20 July 2021: Clinical Research
Risk Prediction Model Based on Biomarkers of Remodeling in Patients with Acute Anterior ST-Segment Elevation Myocardial Infarction
Zeyan Liu12ABCDEF, Lijun Liu1E*, Jinglin Cheng2BCF, Hao Zhang2FGDOI: 10.12659/MSM.927404
Med Sci Monit 2021; 27:e927404
Abstract
BACKGROUND: The aim of the present study was to develop a risk prediction model in patients with acute anterior ST-segment elevation myocardial infarction (STEMI).
MATERIAL AND METHODS: Clinical data from 333 patients with acute anterior STEMI were retrospectively analyzed. Clinical echocardiographic and angiographic data from patients with left ventricular remodeling (LVR) and those without LVR were compared. Factors that influenced risk were identified using multivariate logistic regression analysis. The area under the curve (AUC) of the receiver operating characteristic curve was used to assess the diagnostic performance of the model.
RESULTS: After 6-month follow-up, 135 of the patients experienced LVR (LVR group), whereas 198 did not (non-LVR group). Results of multivariate analysis showed that the number of stenosed coronary vessels, left ventricular end-diastolic volume (LVEDV), left ventricular ejection fraction (LVEF), transforming growth factor-beta (TGF-β) at admission, and cardiac troponin I 3 days after admission (3-d cTnI) were all factors predictive of LVR in patients with acute anterior STEMI (all P<0.05). The established prediction model was Y=-20.639+0.711×number of stenosed coronary vessels + 0.137×LVEDV-0.129×LVEF+0.026×TGF-β at admission + 0.162×3-d cTnI. The estimated AUC of this model was 0.978 (95% confidence interval [CI] 0.955-0.991), significantly superior to the single-factor numbers for stenosed coronary vessel of 0.650 (95% CI 0.597-0.702), LVEDV of 0.876 (95% CI 0.836-0.910), LVEF of 0.684 (95% CI 0.631-0.734), TGF-β at admission of 0.696 (95% CI 0.644-0.745), cTnI at admission of 0.913 (95% CI 0.877-0.941), and 3-d cTnI of 0.945 (95% CI 0.914-0.967).
CONCLUSIONS: The established model had excellent diagnostic accuracy for predicting LVR in patients with acute anterior STEMI.
Keywords: Cardiology, Ventricular Remodeling, Echocardiography, Follow-Up Studies, Predictive Value of Tests, Reproducibility of Results, Risk Assessment, ST elevation myocardial infarction, Transforming Growth Factor beta, troponin I
Background
Acute ST-segment elevation myocardial infarction (STEMI), a severe type of coronary heart disease, seriously affects human health. In Europe, the incidence of STEMI is estimated to range from 43 to 144 per 100 000 per year [1]. It is universally recognized that STEMI is more common in men than in women, and more common in younger than in older patients [2,3]. Primary percutaneous coronary prevention (PPCI) is considered the preferred therapy within 12 h of STEMI symptom onset because it quickly opens coronary arteries, resulting in recovery of myocardial blood supply in the short term [4–6]. However, STEMI-induced myocardial injury can result in left ventricular remodeling (LVR), subsequently leading to changes in LV function and structure [7]. As time goes by, the remodeling adversely influences cardiac function and portends a poor prognosis [8,9].
VR occurs progressively in untreated patients after severe myocardial infarction (MI) [10]. A previous study showed that more severe myocardial injury was related to more severe VR over time [11]. When coronary arteries are occluded, the major factors that determine the area of MI include the infarct-related arterial blood supply range, reperfusion time, and the presence or absence of collaterals, which are usually found to supply retrograde flow to the occluded arteries [12]. At the time of STEMI, the presence of collaterals is associated with extended long-term survival [12]. Clinical experience suggests that, even if patients have no collaterals, the prognosis varies greatly for MI that occurs in different locations. Kiris et al reported that LV function in patients with anterior MI was worse than in those with posterior/inferior MI [13]. Therefore, identification of LVR predictors in patients with anterior STEMI and early prevention may be conductive to improving prognosis in these individuals.
In the present study, we attempted to create a risk prediction model for LVR in patients with acute anterior STEMI, with the aim of early identification of individuals at high risk of LVR and timely treatment of them with the most appropriate regimen to improve their prognosis.
Material and Methods
STUDY POPULATION:
In the present study, clinical data were retrospectively analyzed from 333 patients with acute anterior STEMI treated at the Second Affiliated Hospital of Anhui Medical University between March 2015 and December 2019. All of the patients volunteered to take part in the study, which was approved by the Institutional Review Board of the Second Affiliated Hospital of Anhui Medical University [Approval no. PJ-YX2019-022(F1)].
Inclusion criteria were as follows: (1) age 18 to 80 years; (2) acute anterior STEMI successfully treated with PPCI within the previously noted time window; (3) unilateral left anterior descending artery occlusion and thrombosis in MI flow grade 0 confirmed by coronary angiography; (4) door-to-balloon time <90 min from patient arrival at the hospital gate to coronary guidewire passage through the lesion; and (5) regular use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and/or β receptor blockers after discharge. Exclusion criteria were as follows: (1) right coronary MI, old MI, or cardiogenic shock; (2) complications of myocardiopathy, myocarditis, aortic dissection, rheumatic heart disease, or valvular heart disease; (3) severe cerebrovascular disease, serious liver and kidney dysfunction, infectious disease, immune disease, or tumors; and (4) in ability to participate in regular follow-up.
DATA COLLECTION:
Baseline data from included patients that were systematically collected and sorted via the case management system included age, sex, body mass index (BMI), heart failure classification, marital status, surgical history, and history of drinking, hypertension, diabetes mellitus (DM), or smoking, as well as presence or absence of complications, atrial fibrillation, and ventricular arrhythmia. Information also was collected on laboratory indicators such as transforming growth factor-beta (TGF-β), uric acid (UA), homocysteine (Hcy), cardiac troponin I (cTnI), recombinant human brain natriuretic peptide (rh-BNP), glutamic-pyruvic transaminase (ALT), glutamic oxalacetic transaminase (AST), myoglobin, creatine kinase-myocardial band (CK-MB), and high-sensitivity C-reactive protein (hs-CRP). The SYNTAX score (www.syntaxscore.com) is a unique tool for assessing the complexity of coronary artery disease. Collateral circulation was assessed using Rentrop grade (grade 0: no visible filling of any collateral channel; grade 1: filling of the side branches of the infarct-related artery; grade 2: partial filling of the epicardial vessel of the infarct-related artery; and grade 3: complete collateral filling of the epicardial vessel) [14]. Coronary angiography was performed on all included patients who had complete occlusions of the left anterior descending (LAD) artery. Significant stenosis, defined as ≥50% reduction in coronary artery cross-sectional lumen area, was documented. The most severe stenosis in each of the 2 vessel areas (left circumflex coronary artery [LCX] and right coronary artery) constituted the respective degree of stenosis. Left main coronary artery (LM) stenosis was considered severe stenosis when the coronary artery cross-sectional lumen area was decreased by >50%.
All patients were placed in the left decubitus position. With the body in multiple positions, 3-dimensional echocardiography (Philips EPIC7C) with an echocardiography probe was used to evaluate the size of the heart cavity, including the parasternal short axis, parasternal long axis, apical 4-chamber heart, and apical 2-chamber heart. M-mode ultrasound was used to assess blood flow velocity, the 2-level Simpson method was used to measure EF, and 2- and 3-dimensional ultrasound were utilized to measure peak systolic velocity (PSV) and end-diastolic velocity (EDV). Echocardiogram indicators were recorded at admission, including PSV, EDV, left ventricular end-systolic diameter (LVESD), left ventricular end-systolic volume (LVESV), left ventricular end-diastolic diameter (LVEDD), left ventricular end-diastolic volume (LVEDV), interventricular septum thickness (IVST), posterior wall thickness (PWT), and left ventricular EF (LVEF). LVR was defined as an increase of 20% in LVEDV at 6-month follow-up compared with discharge [15].
STATISTICAL ANALYSIS:
The data were analyzed using SAS software, version 9.4 (SAS Institute Inc., Cary, North Carolina, United States). Normally-distributed data were depicted as means±standard deviations (χ̄±
Results
BASELINE CHARACTERISTICS:
A total of 333 patients with acute anterior STEMI (246 men and 87 women) were included in the present study. The mean age was 61.67±13.17 years. At 6-month follow-up, 135 of the patients (40.54%) had experienced LVR (LVR group), whereas 198 patients (59.46%) had not (non-LVR group).
Baseline characteristics of patients in the LVR and non-LVR groups are listed in Table 1. There were significant differences between the groups in sex (P=0.013), heart failure classification (P<0.001), surgical history (P=0.008), complications (P=0.007), atrial fibrillation (P=0.002), ventricular arrhythmia (P=0.004), number of stenosed coronary vessels (P<0.001), SYNTAX score (P<0.001), and collateral circulation (P<0.001), but not in age, BMI, marital status, history of drinking, hypertension, DM, smoking, or LAD occlusion location (P>0.05).
LABORATORY INDICATORS:
Compared with the non-LVR group, the LVR group had a higher neutrophil/lymphocyte ratio (NLR) (5.57 vs 7.72, t=−3.67, P<0.001); platelet distribution width (PDW) (15.85 vs 16.42, t=−3.17, P=0.002); hemoglobin (142.29 vs 136.33, t=2.73, P=0.007); levels of Hcy (13.04 vs 16.51, t=8.61, P<0.001); and cTnI at admission (2.05 vs 20.10, Z=12.789, P<0.001) and 3 days later (16.26 vs 22..67, Z=4.479, P<0.001); rh-BNP (121.00 vs 454.00, Z=12.213, P<0.001); TGF-β at admission (197.72 vs 215.01, t=5.80, P<0.001), 24 h (257.08 vs 362.73, t=35.54, P<0.001), and 3 days after admission (319.18 vs 463.12, t=35.96, P<0.001); AST (47.00 vs 100.00, Z=4.739, P<0.001); lactic dehydrogenase (LDH) (235.43 vs 538.89, t=10.74, P<0.001); D-dimer (0.25 vs 0.36, Z=2.930, P=0.003); myoglobin (137.20 vs 204.90, Z=7.956, P<0.001); CK-MB (30.50 vs 69.00, Z=10.225, P<0.001); hs-CRP at admission (3.33 vs 11.90, Z=10.697, P<0.001) and 3 days later (6.10 vs 11.30, Z=5.071, P<0.001); and apolipoprotein a (1.31 vs 1.48, t=−3.82, P<0.001), but lower levels of high-density lipoprotein cholesterol (HDL-C) (1.24 vs 1.13, t=2.56, P=0.011). No significant differences were detected between the LVR and non-LVR groups in platelet count, white blood cell (WBC) count, glycosylated hemoglobin, or levels of creatinine, UA, ALT, total bilirubin, fibrinogen, total cholesterol, low-density lipoprotein cholesterol (LDL-C), or apolipoprotein b (P>0.05; Table 2).
ECHOCARDIOGRAM INDICATORS:
The characteristics of the echocardiograms are shown in Table 3. There were significant differences in EDV (0.64 vs 0.66, t=−1.98, P=0.049), LVESD (36.58 vs 38.50, t=0.69, P<0.001), LVESV (52.46 vs 60.03, t=−5.06, P<0.001), LVEDD (47.43 vs 49.18, t=−4.50, P<0.001), LVEDV (121.91 vs 127.49, t=−3.40, P<0.001), and LVEF (60.02 vs 55.51, t=6.24, P<0.001), but no differences in PSV (0.58 vs 0.56, t=0.69, P=0.490), IVST (11.12 vs 10.81, t=1.16, P=0.246), and PWT (9.90 vs 9.63, t=1.56, P=0.121) between the LVR and non-LVR groups.
MULTIVARIATE LOGISTIC REGRESSION ANALYSIS OF LVR:
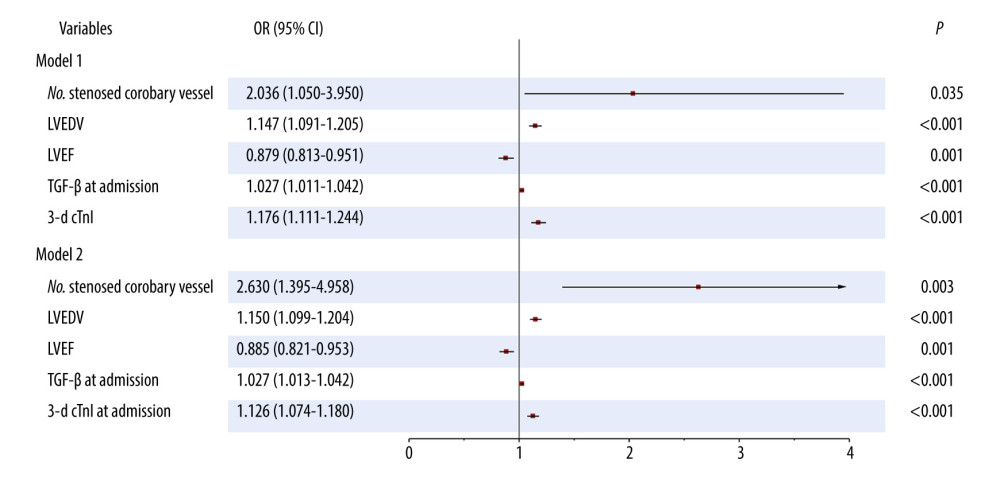
Factors with differences on univariate analysis were further analyzed using multivariate stepwise logistic regression. As seen in Table 4 and Figure 1 (model 1), the number of stenosed coronary vessels (odds ratio [OR] 2.036, 95% CI 1.050–3.950, P=0.035), LVEDV (OR 1.147, 95% CI 1.091–1.205, P<0.001), LVEF (OR 0.879, 95% CI 0.813–0.951, P=0.001), TGF-β at admission (OR 1.027, 95% CI 1.011–1.042, P<0.001), and 3-day cTnI (OR 1.176, 95% CI 1.111–1.244, P<0.001) were identified as factors predictive of LVR in patients with acute anterior STEMI. A logistic regression model was established: Y=−20.639+0.711×number of stenosed coronary vessel+0.137×LVEDV-0.129×LVEF+0.026×TGF-β at admission+0.162×3-d cTnI; P=Y/1+Y. LVR occurred at P>0.5.
In addition, to further explore the stability of the model, the cTnI at admission was replaced with 3-day cTnI. As seen in Table 5 and Figure 1 (model 2), the number of stenosed coronary vessels (OR 2.630, 95% CI 1.395–4.958, P=0.003), LVEDV (OR 1.150, 95% CI 1.099–1.204, P<0.001), LVEF (OR 0.885, 95% CI 0.821–0.953, P=0.001), TGF-β at admission (OR 1.027, 95% CI 1.013–1.042, P<0.001), and cTnI at admission (OR 1.126, 95% CI 1.074–1.180, P<0.001), were identified as factors predictive of LVR. The model was found to be stable, suggesting that it can be used to predict LVR in patients with acute anterior STEMI.
DIAGNOSTIC PERFORMANCE OF PREDICTION MODEL:
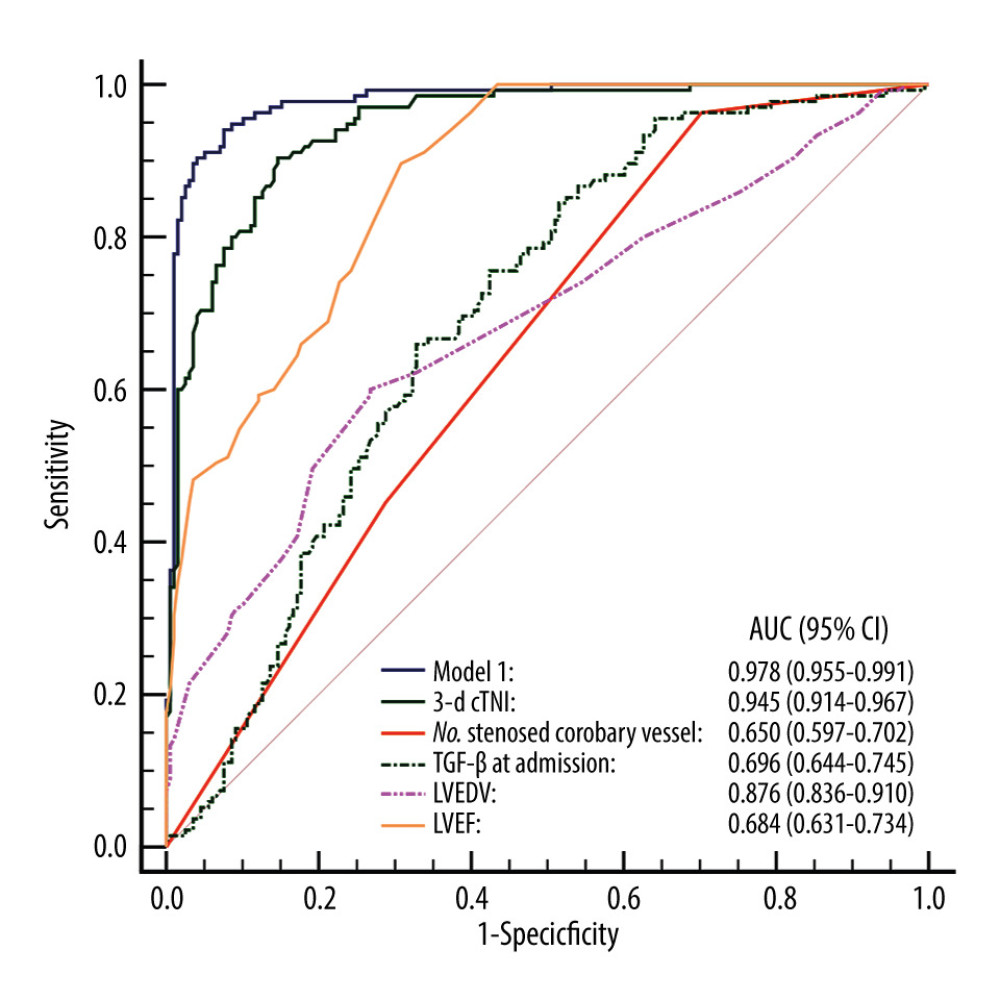
The ROC curves of the prediction model and the single-factor numbers for stenosed coronary vessels, LVEDV, LVEF, TGF-β at admission, and 3-day cTnI were drawn (Figure 2). The results showed that the AUC of the prediction model was 0.978 (95% CI 0.955–0.991) for prediction of LVR risk, similar to the AUC in model 2, which was 0.970 (95% CI 0.946–0.986, P=0.275), and significantly superior to the single-factor numbers for stenosed coronary vessels of 0.650 (95% CI 0.597–0.702, P<0.001), LVEDV of 0.876 (95% CI 0.836–0.910, P<0.001), LVEF of 0.684 (95% CI 0.631–0.734, P<0.001), TGF-β at admission of 0.696 (95% CI 0.644–0.745, P<0.001), cTnI at admission of 0.913 (95% CI 0.877–0.941, P=0.002), and 3-day cTnI of 0.945 (95% CI 0.914–0.967, P<0.001) (Table 6).
Discussion
LVR is usually used to depict the structural, functional, myocellular, and interstitial changes occurring in reaction to myocardial injury and/or chronic changes associated with myocardial loading. It progresses over time in response to increased wall stress, neurohormonal activation, and inflammatory signaling pathways, and is thought to be connected with an elevated risk of primary morbidity and mortality [16]. In the present study, we systematically analyzed clinical data from 333 patients with acute anterior STEMI, including 135 patients with and 1989 patients without LVR. The results of multivariate analysis showed that the numbers for stenosed coronary vessels, LVEDV, LVEF, TGF-β at admission, and 3-day cTnI were the factors predictive of LVR in patients with acute anterior STEMI. Based on these findings, a risk prediction model of LVR was developed: Y=−20.639+0.711×number of stenosed coronary vessels+0.137×LVEDV-0.129×LVEF+0.026×TGF-β at admission+0.162×3-d cTnI;
Cardiac remodeling following MI is characterized by infarct expansion, hypertrophy of surviving myocardium, increased collagen deposition, and chamber structural changes, which eventually lead to heart failure. In the present study, the number of stenosed coronary vessels, LVEDV, LVEF, 3-day cTnI, and TGF-β at admission were all shown to be factors predictive of LVR and included in the model that we created. The results showed that there was a 100% increase in the number of stenosed coronary vessels and a 103.6% increase in the risk of LVR. Early studies reported that impaired diastolic filling was associated with an increasing number of stenosed coronary vessels, and more severe myocardial injury was related to more severe VR over time [11,17]. As a confirmed biomarker for acute MI, cTnI reflects myocardial cell damage with high specificity and sensitivity [18]. By exploring the ability of cTnI to predict LVR after PPCI in STEMI, Hallén et al discovered that for patients with STEMI who receive PPCI, single-point sampling of cTnI could provide critical prognostic information about LV function [19]. In the REVE-2 study, persistently detectable cTnI levels during follow-up were found to be associated with LVR after acute MI [20]. TGF-β, a single-chain peptide composed of 50 amino acid residues, plays an important role in VR through paracrine or autocrine regulation of dermal development, organ formation, and cell proliferation, differentiation, migration, and apoptosis [21]. Expression of TGF-β mRNA in LV myocardium was increased in animal models of MI and pressure overload. Hein et al found that expression of TGF-β was closely related to the degree of myocardial fibrosis in patients with compensatory cardiac hypertrophy that progressed to heart failure [22]. Studies on animal models have shown that TGF-β overexpression causes interstitial fibrosis and cardiac hypertrophy [23]. A consensus from an international forum on cardiac remodeling noted that elevated LVEDV and decreased LVEF occur in post-infarction LVR [24]. Similarly, our findings showed that each 1 mL increase in LVEDV was associated with a 0.147-fold increase, while each 1% increase in LVEF was associated with a 0.121-fold reduction in risk of RV remodeling.
High-quality predictive models are available with which to make clinical decisions and counsel patients and they also may ensure rational allocation of medical resources and improve the design of clinical trials [25]. In the present study, we created a model to predict the risk of LVR in patients with acute anterior STEMI. Our results showed that the AUC of the prediction model was 0.978 for prediction of LVR risk, similar to that for model 2, which was 0.970; therefore, it was significantly superior to the single-factor number for stenosed coronary vessels, LVEDV, LVEF, TGF-β at admission, cTnI at admission and 3-day cTnI. The model appeared to be stable in patients with cTnI at admission and on day 3. To the best of our knowledge, models for predicting LVR in patients with acute anterior STEMI rarely have been described. Hendriks et al explored predictors of LVR after STEMI and created multivariable models [26]. The AUCs of these models were 0.838, 0.799, 0.896, and 0.761, indicating that their predictive efficacy was inferior to our model. The performance of our prediction model was good, suggesting that it can predict LVR in patients with acute anterior STEMI.
The strengths of the present study were that it resulted in a novel risk prediction model for LVR in patients with acute anterior STEMI. The model is simple to use and the required data are accessible in the clinic. A comparison of its diagnostic performance with other single-factor indicators was performed to support the usefulness of the model for discriminating the presence or absence of LVR. In addition, use of the model could provide information essential to determine whether patients are at high risk of LVR and provide timely intervention. The present study, however, also had some limitations, including a relatively small sample size and lack of comprehensive variables, assessment of comorbidities, and validation. In the future, further clinical trials of the model, including validation, will be conducted.
Conclusions
Risk factors for LVR include the number of stenosed coronary vessels, LVEDV, LVEF, TGF-β at admission, and 3-day cTnI. The model we created has excellent diagnostic accuracy for predicting LVR in patients with acute anterior STEMI.
Tables
Table 1. Baseline characteristics in the non-LVR and LVR groups (χ̄±s) or n (%).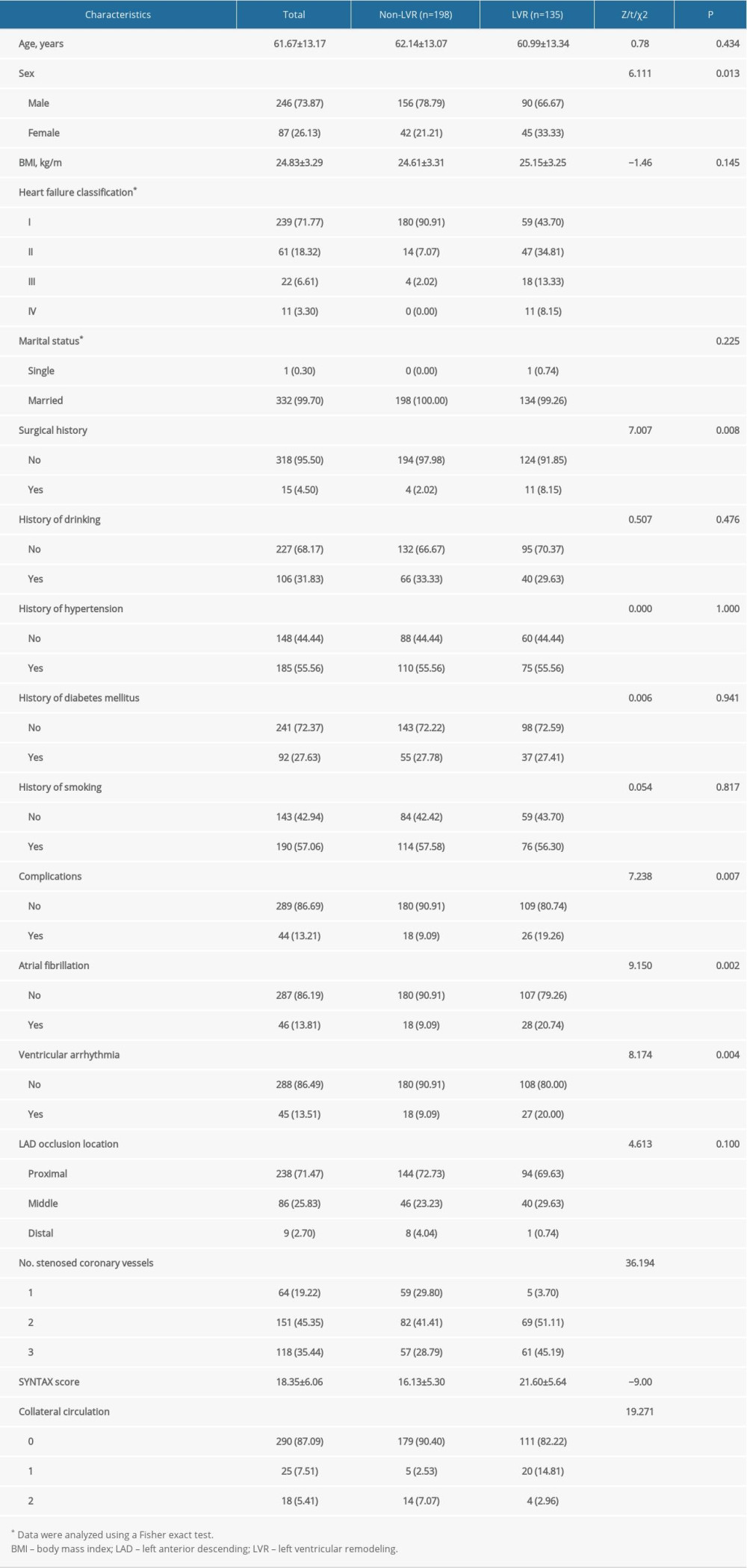 Table 2. Laboratory indicators in the non-LVR and LVR groups, (χ̄±s) or M(Q25, Q75).
Table 2. Laboratory indicators in the non-LVR and LVR groups, (χ̄±s) or M(Q25, Q75).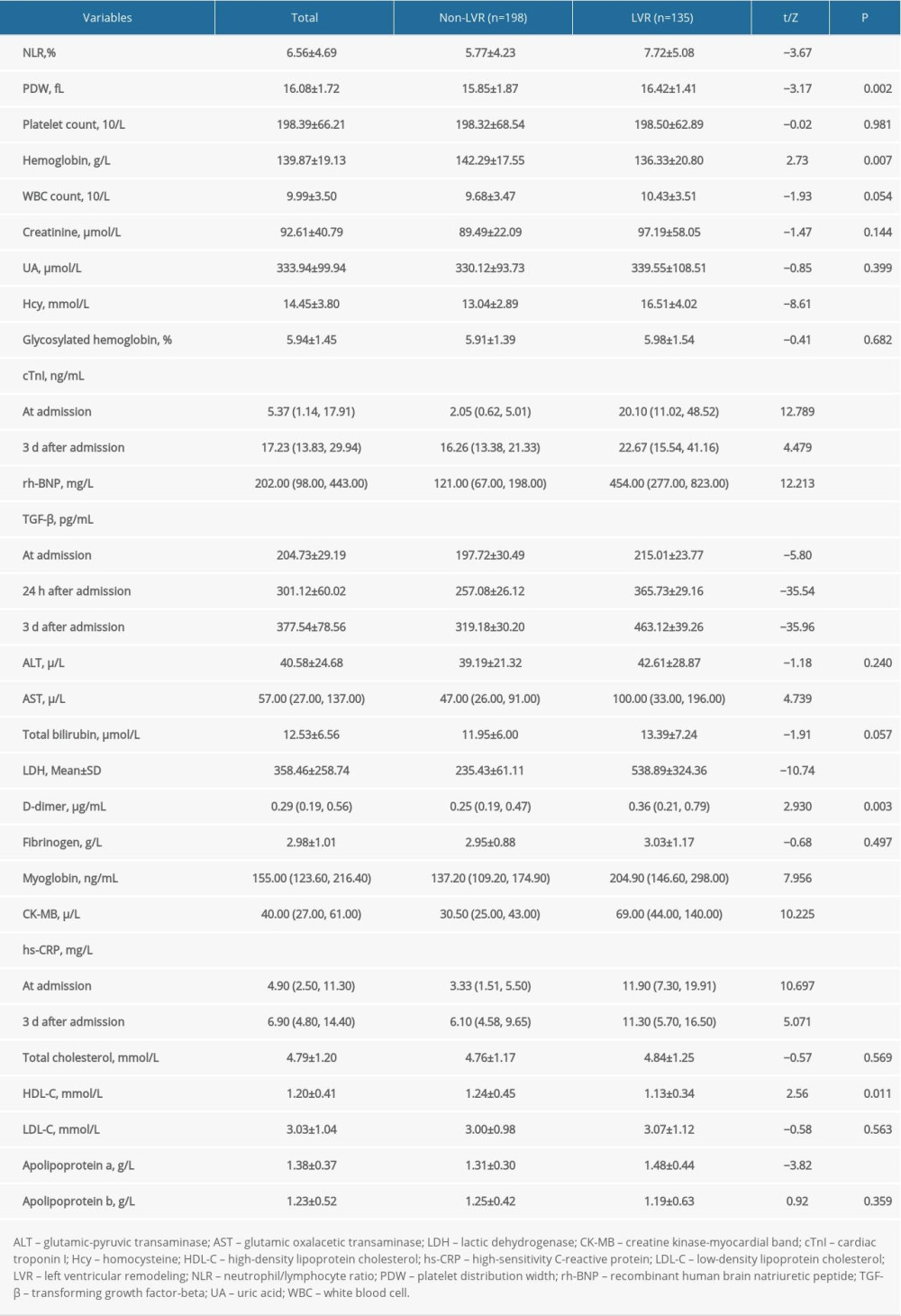 Table 3. Characteristics of cardiac color ultrasound, (χ̄±s).
Table 3. Characteristics of cardiac color ultrasound, (χ̄±s).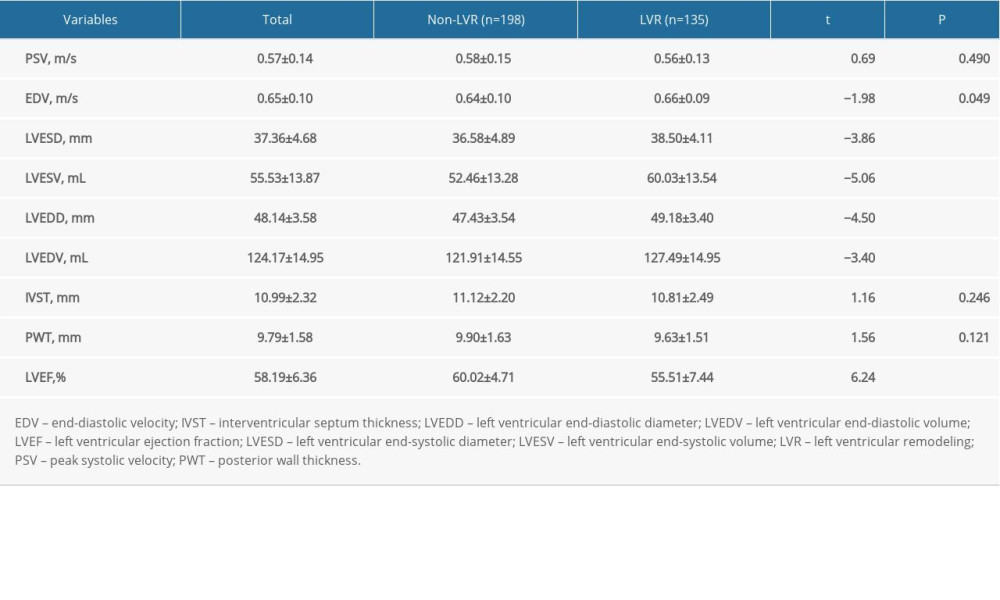 Table 4. Multivariate logistic regression analysis of LVR (model 1).
Table 4. Multivariate logistic regression analysis of LVR (model 1).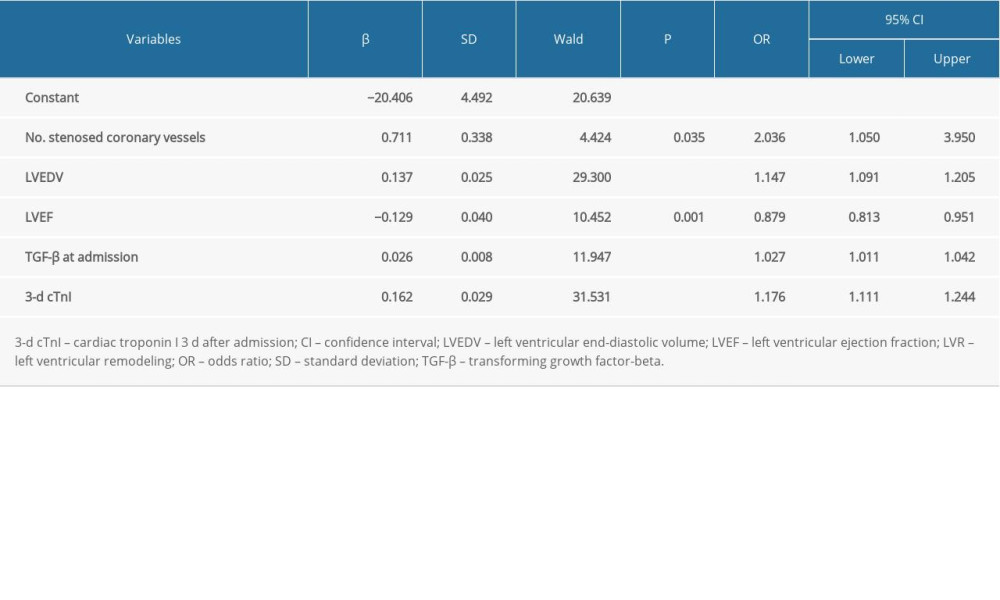 Table 5. Multivariate logistic regression analysis of LVR (model 2).
Table 5. Multivariate logistic regression analysis of LVR (model 2).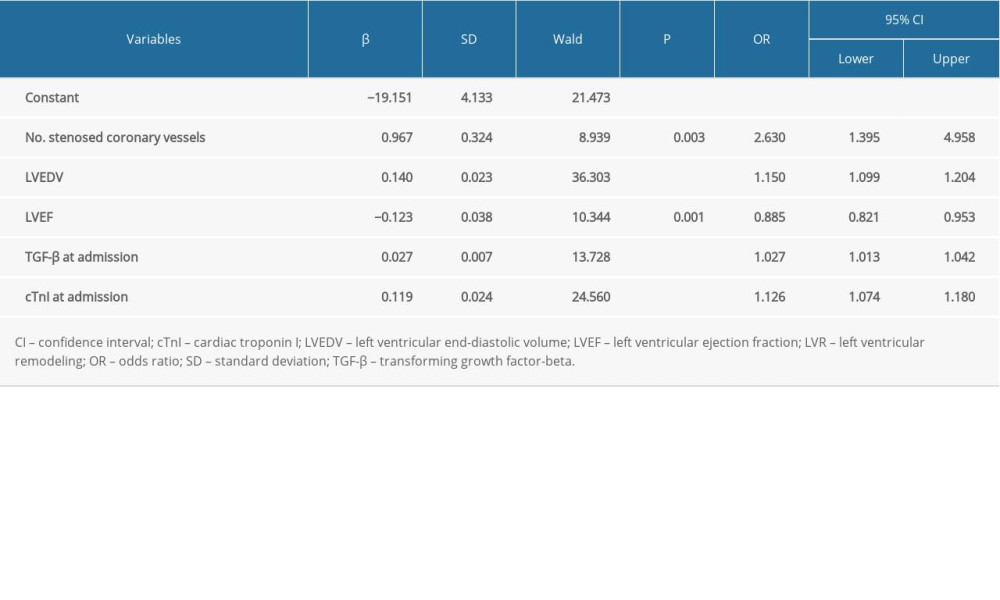 Table 6. Diagnostic performance of the prediction model.
Table 6. Diagnostic performance of the prediction model.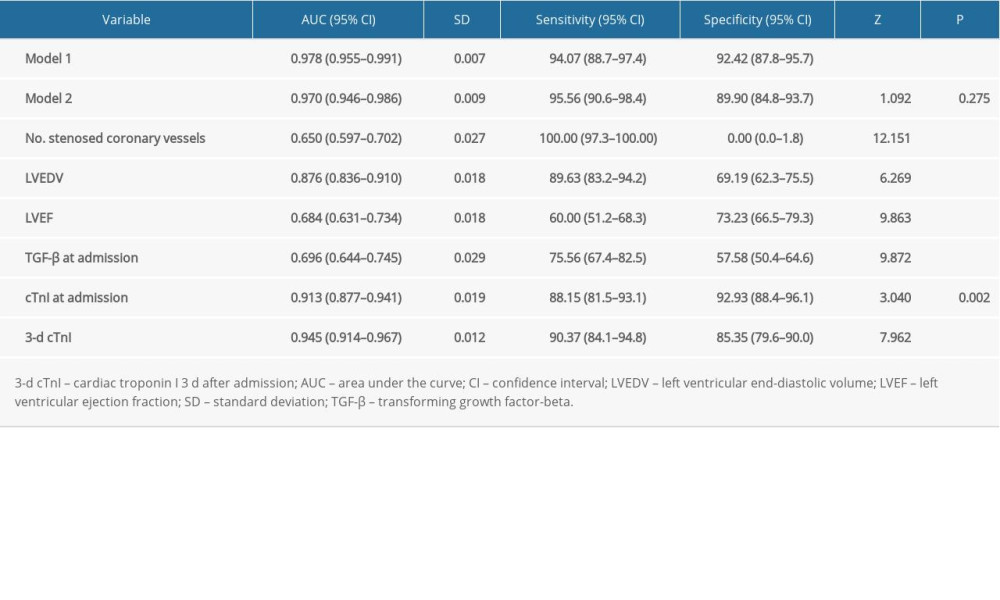
References
1. Widimsky P, Wijns W, Fajadet J, Reperfusion therapy for ST elevation acute myocardial infarction in Europe: Description of the current situation in 30 countries: Eur Heart J, 2010; 31; 943-57
2. Khera S, Kolte D, Gupta T, Temporal trends and sex differences in revascularization and outcomes of ST-segment elevation myocardial infarction in younger adults in the United States: J Am Coll Cardiol, 2015; 66; 1961-72
3. Ibanez B, James S, Agewall S, 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: Kardiol Pol, 2018; 76; 229-313
4. Pedersen F, Butrymovich V, Kelbæk H, Short- and long-term cause of death in patients treated with primary PCI for STEMI: J Am Coll Cardiol, 2014; 64; 2101-8
5. Steg PG, James SKTask Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology (ESC), ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: Eur Heart J, 2012; 33; 2569-619
6. Patel MR, Dehmer GJ, Hirshfeld JW, ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 Appropriate use criteria for coronary revascularization focused update: A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography: J Am Coll Cardiol, 2012; 59; 857-81
7. Cohn JN, Ferrari R, Sharpe N, Cardiac remodeling – concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling: J Am Coll Cardiol, 2000; 35; 569-82
8. Guidry UC, Evans JC, Larson MG, Temporal trends in event rates after Q-wave myocardial infarction: The Framingham Heart Study: Circulation, 1999; 100; 2054-59
9. Jaiswal A, Nguyen VQ, Carry BJ, Pharmacologic and endovascular reversal of left ventricular remodeling: J Card Fail, 2016; 22; 829-39
10. Konstam MA, Kramer DG, Patel AR, Left ventricular remodeling in heart failure: Current concepts in clinical significance and assessment: JACC Cardiovasc Imaging, 2011; 4; 98-108
11. Pfeffer MA, Braunwald E, Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications: Circulation, 1990; 81; 1161-72
12. Alsanjari O, Chouari T, Williams T, Angiographically visible coronary artery collateral circulation improves prognosis in patients presenting with acute ST segment-elevation myocardial infarction: Catheter Cardiovasc Interv, 2020; 96; 528-33
13. Kiris I, Kapan S, Narin C, Relationship between site of myocardial infarction, left ventricular function and cytokine levels in patients undergoing coronary artery surgery: Cardiovasc J Afr, 2016; 27; 299-306
14. Rentrop KP, Cohen M, Blanke H, Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects: J Am Coll Cardiol, 1985; 5; 587-92
15. Urbano-Moral JA, Lopez-Haldon JE, Fernandez M, Prognostic value of different serum biomarkers for left ventricular remodeling after ST-elevation myocardial infarction treated with primary percutaneous coronary intervention: Heart, 2012; 98; 1153-59
16. Saraon T, Katz SD, Reverse remodeling in systolic heart failure: Cardiol Rev, 2015; 23; 173-81
17. Rydberg E, Willenheimer R, Erhardt L, The prevalence of impaired left ventricular diastolic filling is related to the extent of coronary atherosclerosis in patients with stable coronary artery disease: Coron Artery Disease, 2002; 13; 1-7
18. Zhu R, Sun H, Yu K, Interleukin-37 and dendritic cells treated with interleukin-37 plus Troponin I ameliorate cardiac remodeling after myocardial infarction: J Am Heart Assoc, 2016; 5; e004406
19. Hallén J, Jensen JK, Fagerland MW, Cardiac troponin I for the prediction of functional recovery and left ventricular remodelling following primary percutaneous coronary intervention for ST-elevation myocardial infarction: Heart, 2010; 96; 1892-97
20. Fertin M, Hennache B, Hamon M, Usefulness of serial assessment of B-type natriuretic peptide, troponin I, and C-reactive protein to predict left ventricular remodeling after acute myocardial infarction (from the REVE-2 study): Am J Cardiol, 2010; 106; 1410-16
21. Bujak M, Frangogiannis NG, The role of TGF-beta signaling in myocardial infarction and cardiac remodeling: Cardiovasc Res, 2007; 74; 184-95
22. Hein S, Arnon E, Kostin S, Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: Structural deterioration and compensatory mechanisms: Circulation, 2003; 107; 984-91
23. Yao Y, Hu C, Song Q, ADAMTS16 activates latent TGF-β, accentuating fibrosis and dysfunction of the pressure-overloaded heart: Cardiovasc Res, 2020; 116; 956-69
24. Cohn JN, Ferrari R, Sharpe N, Cardiac remodeling – concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling: J Am Coll Cardiol, 2000; 35; 569-82
25. EWS: Clinical prediction models: A practical approach to development, validation and updating, 2009, New York, NY, Springer
26. Hendriks T, Hartman MHT, Vlaar PJJ, Predictors of left ventricular remodeling after ST-elevation myocardial infarction: Int J Cardiovasc Imaging, 2017; 33; 1415-23
Figures
Tables
 Table 1. Baseline characteristics in the non-LVR and LVR groups (χ̄±s) or n (%).
Table 1. Baseline characteristics in the non-LVR and LVR groups (χ̄±s) or n (%). Table 2. Laboratory indicators in the non-LVR and LVR groups, (χ̄±s) or M(Q25, Q75).
Table 2. Laboratory indicators in the non-LVR and LVR groups, (χ̄±s) or M(Q25, Q75). Table 3. Characteristics of cardiac color ultrasound, (χ̄±s).
Table 3. Characteristics of cardiac color ultrasound, (χ̄±s). Table 4. Multivariate logistic regression analysis of LVR (model 1).
Table 4. Multivariate logistic regression analysis of LVR (model 1). Table 5. Multivariate logistic regression analysis of LVR (model 2).
Table 5. Multivariate logistic regression analysis of LVR (model 2). Table 6. Diagnostic performance of the prediction model.
Table 6. Diagnostic performance of the prediction model. Table 1. Baseline characteristics in the non-LVR and LVR groups (χ̄±s) or n (%).
Table 1. Baseline characteristics in the non-LVR and LVR groups (χ̄±s) or n (%). Table 2. Laboratory indicators in the non-LVR and LVR groups, (χ̄±s) or M(Q25, Q75).
Table 2. Laboratory indicators in the non-LVR and LVR groups, (χ̄±s) or M(Q25, Q75). Table 3. Characteristics of cardiac color ultrasound, (χ̄±s).
Table 3. Characteristics of cardiac color ultrasound, (χ̄±s). Table 4. Multivariate logistic regression analysis of LVR (model 1).
Table 4. Multivariate logistic regression analysis of LVR (model 1). Table 5. Multivariate logistic regression analysis of LVR (model 2).
Table 5. Multivariate logistic regression analysis of LVR (model 2). Table 6. Diagnostic performance of the prediction model.
Table 6. Diagnostic performance of the prediction model. In Press
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952