21 December 2020: Clinical Research
Urinary Liver-Type Fatty Acid-Binding Protein Predicts Residual Renal Function Decline in Patients on Peritoneal Dialysis
Kenta Torigoe1ABCDEF, Kumiko Muta1ABCDEF*, Kiyokazu Tsuji1B, Ayuko Yamashita1B, Yuki Ota1B, Mineaki Kitamura1DE, Hiroshi Mukae2G, Tomoya Nishino1ADEGDOI: 10.12659/MSM.928236
Med Sci Monit 2020; 26:e928236
Abstract
BACKGROUND: Liver-type fatty acid-binding protein (L-FABP) is a predictive marker for the early detection of acute kidney injury; however, less is known about how useful it is for predicting residual renal function (RRF) decline in patients on peritoneal dialysis (PD).
MATERIAL AND METHODS: The study subjects were 35 patients on PD who underwent multiple peritoneal equilibration tests (PETs) between October 2011 and October 2019. Urinary L-FABP levels were analyzed with enzyme-linked immunosorbent assay. The relationship between baseline clinical data, including urinary L-FABP levels and the subsequent annual rate of renal Kt/V decline, was investigated.
RESULTS: The median follow-up duration was 11 months and the rate of renal Kt/V decline was 0.29/y. Compared with outcomes in the group with renal Kt/V preservation, renal Kt/V decline was associated with both high daily levels of urinary protein excretion (0.60 g/d [range, 0.50–0.87] vs. 0.36 g/d [range, 0.19–0.48]; P=0.01) and high daily levels of urinary L-FABP excretion (111.2 mg/d [range, 76.1–188.6] vs. 61.5 mg/d [range, 35.7–96.0]; P=0.002). Multiple logistic regression analysis showed that only high daily levels of urinary L-FABP excretion were independently associated with renal Kt/V decline (odds ratio 1.03, 95% confidence interval 1.00–1.05; P=0.001). Furthermore, higher daily levels of urinary L-FABP excretion were significantly correlated with the higher annual rate of renal Kt/V decline (r=0.71, P<0.001).
CONCLUSIONS: We demonstrated that daily levels of urinary L-FABP are associated with RRF decline in patients on PD. The results of the present study indicate that assessment of urinary L-FABP levels may help predict RRF decline in patients on PD.
Keywords: Fatty Acid-Binding Proteins, Kidney Failure, Chronic, Peritoneal Dialysis, Disease Progression, Kidney, Kidney Function Tests
Background
Peritoneal dialysis (PD) is one of the dialytic therapies for patients with end-stage renal disease (ESRD). There are some differences between PD and hemodialysis (HD) in terms of technical demand, frequency of hospital visits, and effects on hemodynamics. In particular, PD is reported to be associated with greater preservation of residual renal function (RRF) [1,2].
In patients with ESRD, including those on HD and PD, preservation of RRF is an important factor because RRF decline is associated with various unfavorable outcomes, including all-cause mortality [3–5]. Furthermore, in patients on PD, loss of RRF is associated with withdrawal from PD [1,6]. Therefore, identification of risk factors for RRF decline is important. Previous studies have reported that male sex, high body mass index, advanced stages of congestive heart failure, low blood pressure, and high 24-h proteinuria values are the factors associated with faster RRF decline in patients on PD [7,8]. However, there is no established marker for predicting RRF decline in patients on PD.
Liver-type fatty acid-binding protein (L-FABP) is a carrier protein that plays a role in intracellular transport of free fatty acids [9]. It is expressed in proximal tubular cells in the kidney and secreted into the urine when tubular damage occurs. Therefore, levels of urinary L-FABP are used as a marker for acute kidney injury (AKI) in clinical settings in Japan [10–12]. Some studies have reported the utility of urinary L-FABP levels in AKI and other renal diseases. In patients with chronic kidney disease (CKD), urinary L-FABP levels are associated with progression of renal failure and reflect renal interstitial fibrosis, an important factor in renal prognosis [13–15]. However, few studies have reported on the utility of urinary L-FABP levels in patients on PD. Furthermore, no study has investigated the association between urinary L-FABP levels and RRF decline in patients on PD. Therefore, in the present study, we aimed to investigate whether urinary L-FABP levels are associated with RRF decline in patients on PD.
Material and Methods
STUDY SUBJECTS:
In the present retrospective study, we enrolled 35 patients who underwent PD in a university hospital between October 2011 and October 2019 and were given a peritoneal equilibration test (PET) more than once. We excluded patients who had anuria (daily urine volume <200 mL) and those who underwent both PD and HD at the time of the first PET done during the study period. This study was approved by the Ethics Committee of Nagasaki University Hospital (approval number: 09022759-3) and performed in accordance with the ethical standards set forth in the Declaration of Helsinki and its later amendments.
DATA COLLECTION:
Patient baseline clinical data were collected at the time of first PET during the study period. We also collected 24-h urine samples. Renal Kt/V value of urea, which reflects residual renal urea clearance, was used as a marker of RRF. The annual rate of renal Kt/V decline was calculated according to the following formula: (renal Kt/V at first PETrenal Kt/V at the next PET)/number of years of follow-up.
ANALYSIS OF URINARY L-FABP:
Analysis of urinary L-FABP was performed using the 24-h urine sample at the time of first PET during the study period. We used the Human L-FABP ELISA Kit (CMIC Holdings Co., Ltd., Tokyo, Japan) in accordance with the manufacturer’s instructions. The absorbance was measured at 450 nm with a microplate reader (Multiskan FC; Thermo Scientific, Kanagawa, Japan). All samples were analyzed in duplicate.
STATISTICAL ANALYSIS:
Categorical variables are expressed as numbers or percentages. Continuous variables are expressed as means±standard deviations and non-normally distributed data are expressed as medians and interquartile ranges. To retrospectively investigate the clinical parameters associated with annual rate of renal Kt/V decline, we divided patients on PD into 2 groups according to the median value of the annual rate of renal Kt/V decline. A Fisher exact test or Pearson chi-square test was conducted for nominal variables and a Wilcoxon rank sum test was conducted for continuous variables when comparing the groups. Multivariate logistic regression analysis was performed to investigate the factors independently associated with renal Kt/V decline. Simple regression analysis was conducted to investigate the relationship between the annual rate of renal Kt/V decline and clinical parameters, and multivariate regression analysis was performed to investigate the factors independently associated with the annual rate of renal Kt/V decline. All statistical analyses were performed using JMP software, version 13 (SAS Institute Inc., Cary, North Carolina, U.S.A.).
Results
PATIENT CHARACTERISTICS:
Table 1 lists characteristics at first PET of patients on PD. The median age was 61.1±12.9 years, and 20 patients (57%) were men. The median duration of PD was 13 months (range, 11–24). Nephrosclerosis was the main cause of ESRD (34.3%) and 25.7% of patients had diabetes mellites. Urine volume was preserved (1500 mL; range, 1120–1700), and total Kt/V was 2.00 (range, 1.67–2.49), which reflects adequate small solute clearance. Values for excretion of urinary protein and L-FABP were 0.51 g/d (range, 0.26–0.76) and 82.8 μg/d (range, 59.9–125.9), respectively. Renal Kt/V at first PET was 0.85 (range, 0.64–1.45) and the subsequent annual rate of renal Kt/V decline was 0.29/y (range, 0.05–0.48), with a median duration of renal Kt/V at follow-up of 11 months (range, 11–12).
FACTORS ASSOCIATED WITH RRF DECLINE IN PATIENTS ON PD:
Next, we investigated clinical factors associated with renal Kt/V decline in patients on PD. We divided them into 2 groups, based on the median value of their annual rate of renal Kt/V decline, and compared the clinical parameters between the 2 groups. The median value for the annual rate of renal Kt/V decline was 0.29/y among the study population. Therefore, patients on PD were divided into a group with preserved renal Kt/V (annual rate of renal Kt/V decline <0.29/y, n=17) and a group with renal Kt/V decline (annual rate of renal Kt/V decline ≥0.29, n=18). As shown in Table 2, renal Kt/V decline was associated with both high daily levels of urinary protein excretion (0.60 g/d; range, 0.50–0.87 vs. 0.36 g/d; range, 0.19–0.48; P=0.01) and high daily levels of urinary L-FABP excretion (111.2 μg/d; range, 76.1–188.6 vs. 61.5 μg/d; range, 35.7–96.0; P=0.002). However, other clinical parameters, such as comorbidity and medication (including renin-angiotensin system inhibitor), did not show an association with renal Kt/V decline. Furthermore, as shown in Table 3, multiple logistic regression analysis showed that only high daily levels of urinary L-FABP excretion were independently associated with renal Kt/V decline (odds ratio 1.03, 95% confidence interval, 1.00–1.05; P=0.001). In contrast, daily urinary protein excretion was not associated with renal Kt/V decline.
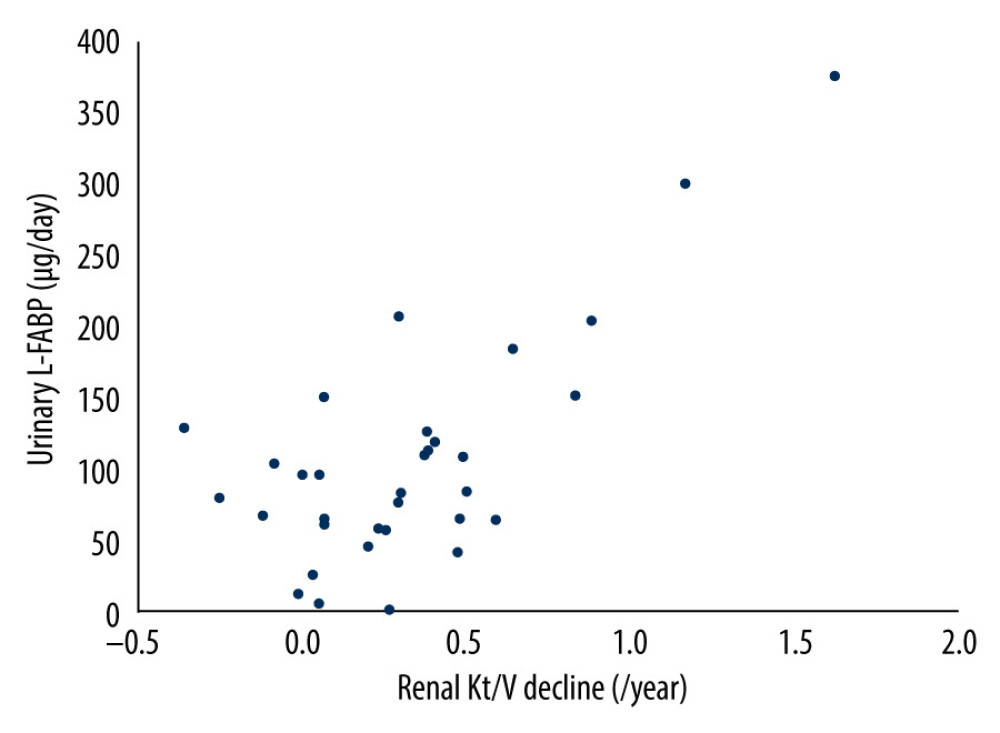
Next, we investigated the correlation between the annual rate of renal Kt/V decline and daily levels of urinary protein excretion or of urinary L-FABP excretion. As shown in Table 4 and the Figure 1, in simple regression analysis, only daily urinary L-FABP excretion was significantly positively correlated with the annual rate of renal Kt/V decline (r=0.71, P<0.001). Furthermore, multiple regression analysis also showed that only daily urinary L-FABP excretion was independently correlated with the annual rate of renal Kt/V decline (Table 4).
Discussion
In the present study, we investigated the significance of urinary L-FABP in patients on PD and demonstrated that higher daily urinary levels of L-FABP were associated with RRF decline. However, daily urinary protein excretion was not associated with RRF decline in patients on PD.
In basic and clinical studies, urinary L-FABP has been reported to be a marker for renal injury in various renal diseases, such as AKI and CKD. L-FABP is expressed in proximal tubular cells and protects against tubular injury by oxidative stress [16]. The promoter region of human L-FABP contains hypoxia-inducible factor-1; thus, hypoxia induces L-FABP expression, following which, L-FABP acts as a renal protective agent by binding to lipid peroxide and is excreted in urine [17,18]. Therefore, in patients with AKI, urinary L-FABP levels increase before serum creatinine; the usefulness of L-FABP in early diagnosis and in predicting the severity of AKI has been reported [19,20]. In patients with CKD who are not on dialysis, previous studies have reported that urinary L-FABP is associated with renal interstitial fibrosis and aids in renal prognosis [13–15]. Renal interstitial fibrosis is induced by various factors. Urinary protein excretion is one of the causes of tubular damage that stimulates urinary L-FABP secretion [13,21]. However, levels of urinary L-FABP also are elevated in patients with CKD who are not on dialysis and do not have albuminuria, suggesting that damage to renal tubular cells is caused not only by urinary protein but also by various conditions, such as ischemia and oxidative stress [22]. It is unclear whether the mechanism of RRF decline is exactly the same for patients on PD and those with CKD who are not on dialysis. However, tubulointerstitial fibrosis is the best predictor of renal prognosis in patients with CKD who are not on dialysis, and it may be similar in patients on PD [23]. Therefore, we speculated that urinary L-FABP predicted RRF decline by reflecting tubulointerstitial injury, which induces tubulointerstitial fibrosis, in patients on PD as well as patients with CKD who are not on dialysis. In contrast, previous reports have investigated the association between various types of tubular injury markers and RRF decline in patients on PD, but some markers have demonstrated no association [24]. Some tubular injury markers are excreted in the urine as a result of renal tubular cell damage; however, L-FABP is excreted in urine in response to cellular stress prior to renal tubular cell damage [25]. Therefore, it was assumed that urinary L-FAPB was able to predict subsequent RRF decline in patients on PD.
Various factors have been investigated to determine the relationship between different markers of RRF decline in patients on PD. A new biomarker is needed, in particular, to predict RRF decline so that the practice of PD can be improved. Urinary protein has been reported to be associated with RRF decline in patients on PD [8]. However, in the present study, multivariate analysis did not show any association between urinary protein excretion and RRF decline. Urinary protein is a marker that mainly reflects glomerular disorders [26]. As described above, urinary L-FABP levels are a marker of tubulointerstitial injury caused by various factors, including glomerular filtered proteinuria. In patients with CKD who are not on dialysis, renal prognosis is reflected more by tubulointerstitial fibrosis than by glomerular sclerosis [27]. Therefore, urinary protein may be associated with RRF decline in a small proportion of patients on PD, whereas urinary L-FABP levels are speculated to reflect RRF decline in a wide proportion of patients on PD.
Prediction of RRF decline is an important factor in patients on PD and the present study showed the usefulness of urinary L-FABP as a prognostic factor for such decline. However, even if L-FABP levels can predict RRF decline, it is important to determine whether urinary L-FABP excretion can be modified and whether improvement in urinary L-FABP excretion can lead to preservation of RRF in patients on PD. Few studies have investigated this issue in patients on PD; however, a previous study reported that urinary L-FABP excretion was decreased in patients on PD who underwent exercise therapy [16]. Moreover, in patients with CKD, previous studies have reported that drugs such as telmisartan and enalapril reduce urinary L-FABP excretion [28,29]. Therefore, it must be possible to reduce urinary L-FABP excretion in patients on PD using some intervention. At present, however, it remains unclear whether treating urinary L-FABP excretion leads to preservation of RRF in patients on PD. Future prospective studies are needed to investigate this issue.
The present study has several limitations, including those mentioned above. First, the sample size was small and it was retrospective and observational. Accordingly, the existence of unrecognized confounding factors that affect RRF decline cannot be completely excluded. Furthermore, the small sample size limited the number of independent variables in multivariate analysis. Second, we used residual renal Kt/V as an indicator of RRF. Renal Kt/V was calculated by determining renal urea clearance, which reflects the clearance of small molecules. Thus, our results may not reflect true RRF, including the clearance of medium-to-large molecules. Third, we calculated RRF decline over a short duration (median duration of follow-up: 11 months). Thus, our results may not reflect long-term RRF decline. Fourth, in our study, we did not consider other urinary biomarkers apart from urinary protein for comparison. Therefore, it is unclear whether urinary L-FABP levels have more predictive value for RRF decline than other existing urinary biomarkers.
Conclusions
We demonstrated that daily urinary L-FABP levels are associated with RRF decline in patients on PD. The results of the present study suggest that assessment of urinary L-FABP may help predict RRF decline in these patients.
Tables
Table 1. Characteristics of patients on peritoneal dialysis.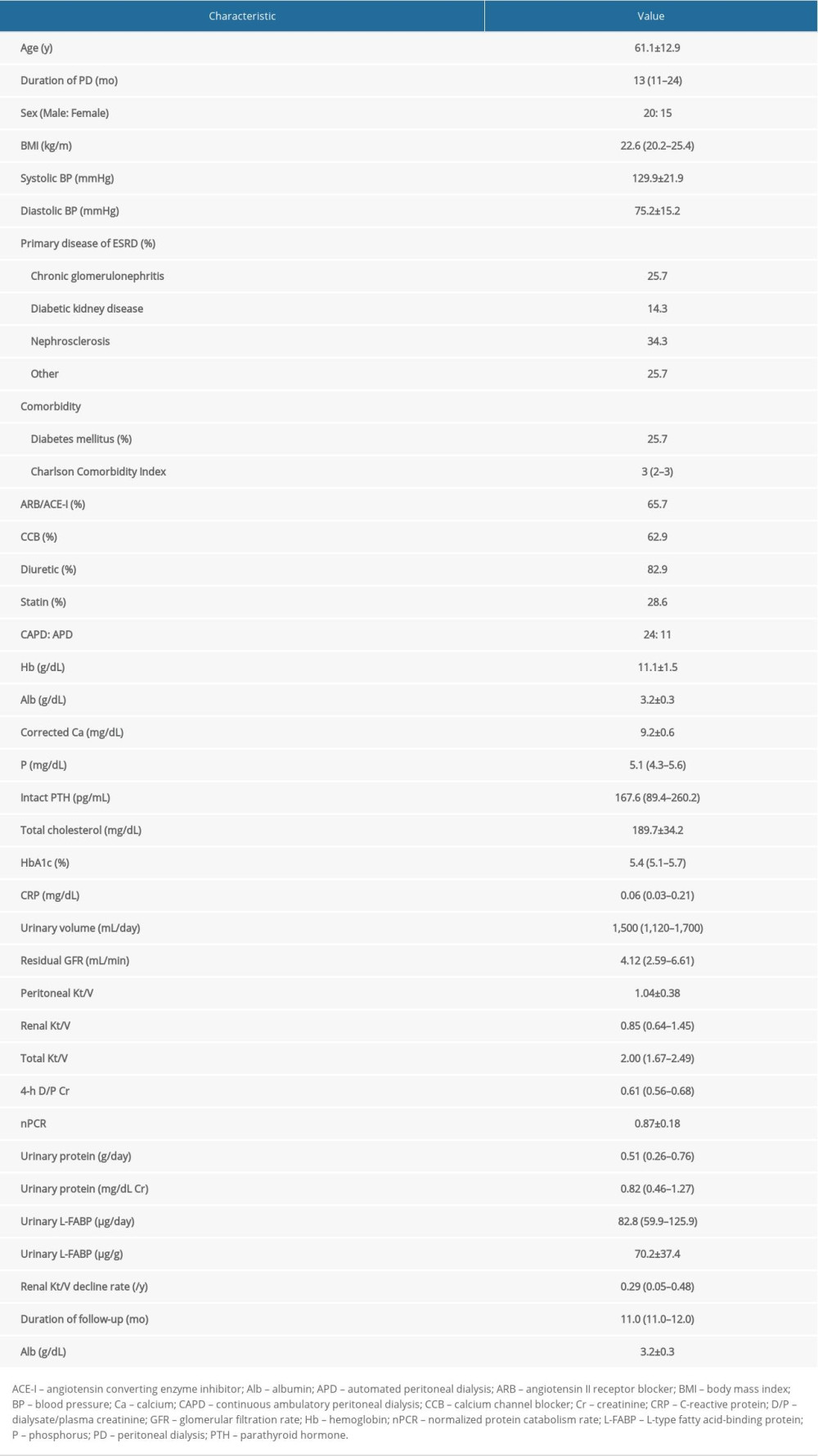 Table 2. Comparison of clinical characteristics between RRF preservation and decline groups.
Table 2. Comparison of clinical characteristics between RRF preservation and decline groups.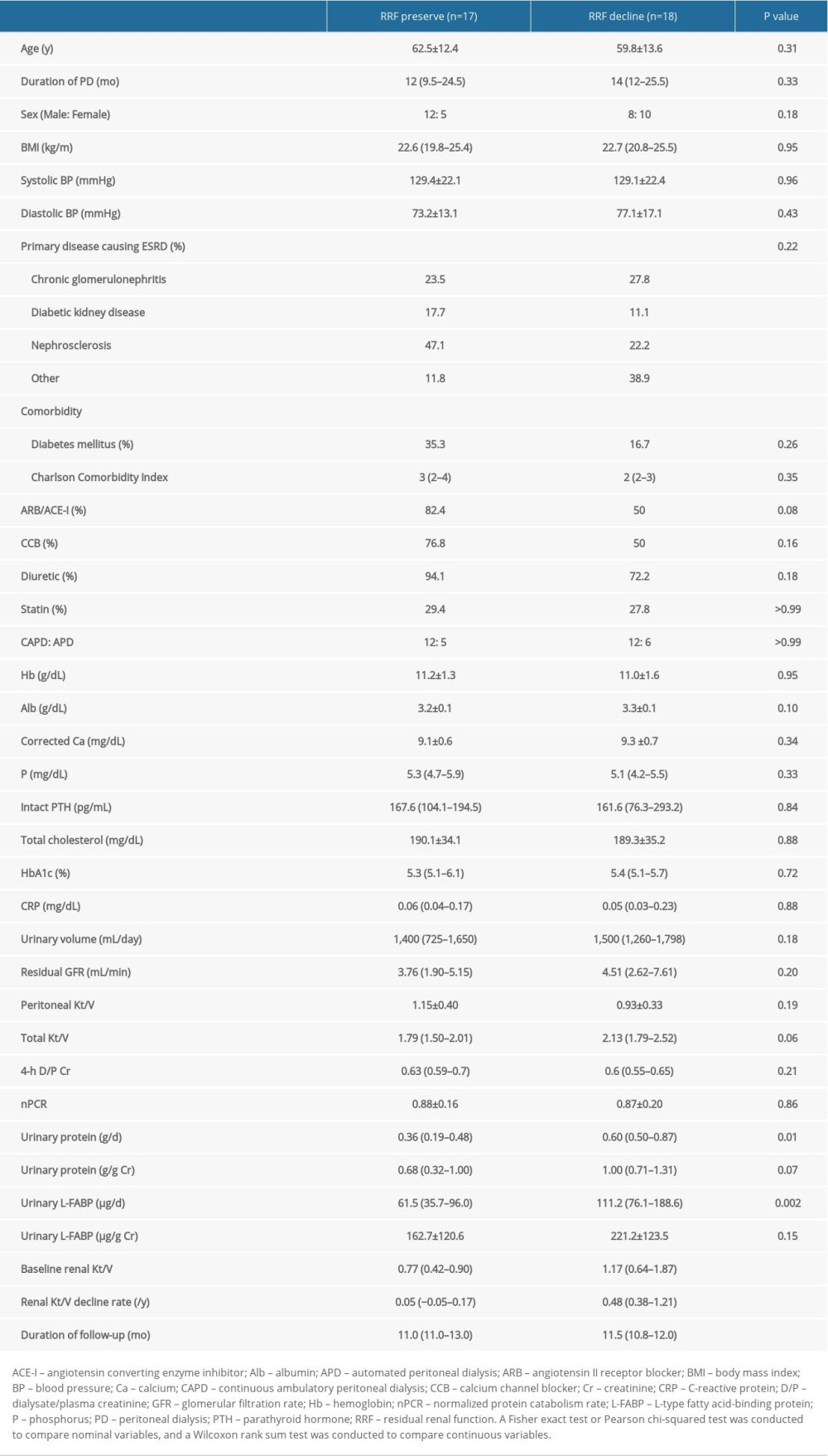 Table 3. Multiple logistic regression analysis of RRF decline in patients on PD.
Table 3. Multiple logistic regression analysis of RRF decline in patients on PD.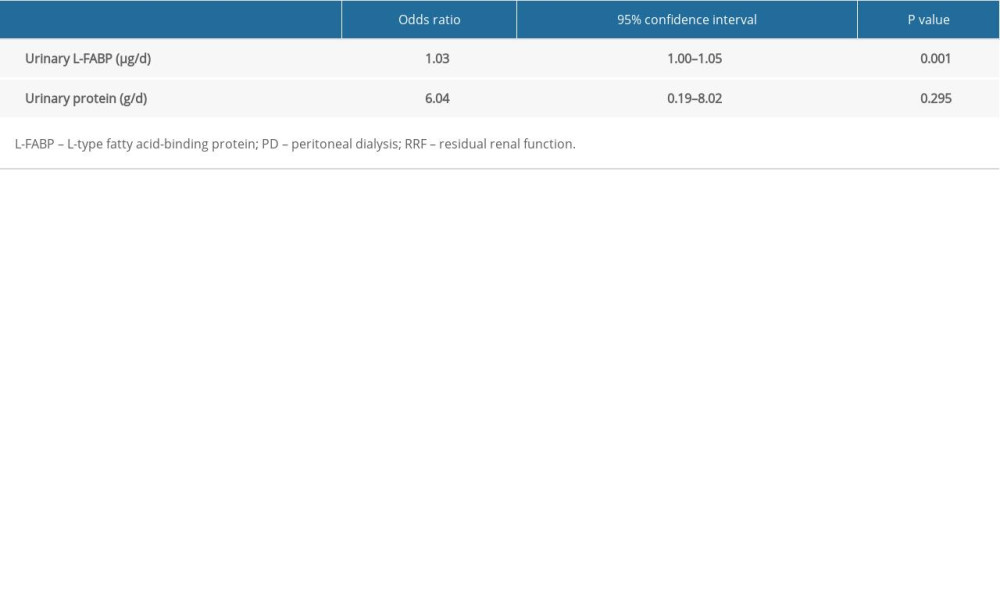 Table 4. Simple and multiple regression analyses of the rate of RRF decline in patients on PD.
Table 4. Simple and multiple regression analyses of the rate of RRF decline in patients on PD.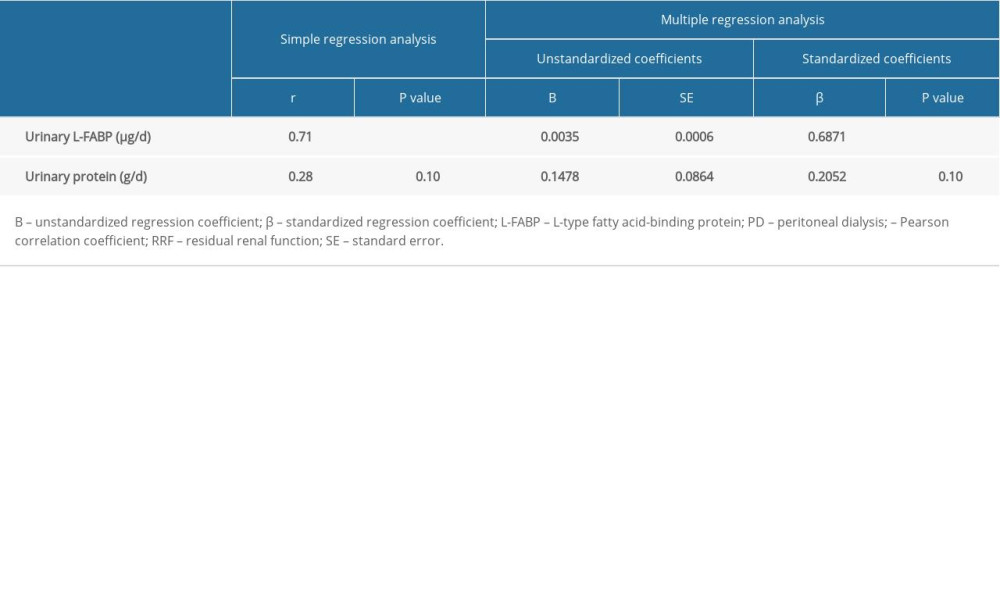
References
1. Wang J, Xie X, Yan X, A fast decline of residual renal function in the first year is a predictor for early withdrawal from peritoneal dialysis in non-diabetic patients: Kidney Blood Press Res, 2019; 44; 12-21
2. Sukul N, Zhao J, Fuller DS, Patient-reported advantages and disadvantages of peritoneal dialysis: Results from the PDOPPS: BMC Nephrol, 2019; 20(1); 116
3. Obi Y, Rhee CM, Mathew AT, Residual kidney function decline and mortality in incident hemodialysis patients: J Am Soc Nephrol, 2016; 27; 3758-68
4. Wang AYM, Lai KN, The importance of residual renal function in dialysis patients: Kidney Int, 2006; 69; 1726-32
5. Marrón B, Remón C, Pérez-Fontán M, Benefits of preserving residual renal function in peritoneal dialysis: Kidney Int Suppl, 2008; 73; S42-51
6. Chaudhary K, Peritoneal dialysis drop-out: Causes and prevention strategies: Int J Nephrol, 2011; 2011; 434608
7. Uchiyama K, Yanai A, Maeda K, Baseline and time-averaged values predicting residual renal function decline rate in Japanese peritoneal dialysis patients: Ther Apher Dial, 2017; 21; 599-605
8. Singhal MK, Bhaskaran S, Vidgen E, Rate of decline of residual renal function in patients on continuous peritoneal dialysis and factors affecting it: Perit Dial Int, 2000; 20; 429-38
9. Maatman RGHJ, van de Westerlo EM, van Kuppevelt TH, Molecular identification of the liver- and the heart-type fatty acid-binding proteins in human and rat kidney. Use of the reverse transcriptase polymerase chain reaction: Biochem J, 1992; 288; 285-90
10. Nakamura T, Sugaya T, Node K, Urinary excretion of liver-type fatty acid-binding protein in contrast medium-induced nephropathy: Am J Kidney Dis, 2006; 47; 439-44
11. Yamamoto T, Noiri E, Ono Y, Renal L-type fatty acid – binding protein in acute ischemic injury: J Am Soc Nephrol, 2007; 18; 2894-902
12. Schrezenmeier EV, Barasch J, Budde K, Biomarkers in acute kidney injury – pathophysiological basis and clinical performance: Acta Physiol (Oxf), 2017; 219; 554-72
13. Kamijo A, Kimura K, Sugaya T, Urinary fatty acid-binding protein as a new clinical marker of the progression of chronic renal disease: J Lab Clin Med, 2004; 143; 23-30
14. Kamijo A, Sugaya T, Hikawa A, Clinical evaluation of urinary excretion of liver-type fatty acid-binding protein as a marker for the monitoring of chronic kidney disease: A multicenter trial: J Lab Clin Med, 2005; 145; 125-33
15. Sasaki H, Kamijo-Ikemori A, Sugaya T, Urinary fatty acids and liver-type fatty acid binding protein in diabetic nephropathy: Nephron Clin Pract, 2009; 112; c148-56
16. Matsui K, Kamijo-Ikemorif A, Sugaya T, Renal liver-type fatty acid binding protein (L-FABP) attenuates acute kidney injury in aristolochic acid nephrotoxicity: Am J Pathol, 2011; 178; 1021-32
17. Noiri E, Doi K, Negishi K, Urinary fatty acid-binding protein 1: An early predictive biomarker of kidney injury: Am J Physiol Renal Physiol, 2009; 296; F669-79
18. Sato E, Kamijo-Ikemori A, Oikawa T, Urinary excretion of liver-type fatty acid-binding protein reflects the severity of sepsis: Ren Replace Ther, 2017; 3; 26
19. Portilla D, Dent C, Sugaya T, Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery: Kidney Int, 2008; 73; 465-72
20. Negishi K, Noiri E, Doi K, Monitoring of urinary L-type fatty acid-binding protein predicts histological severity of acute kidney injury: Am J Pathol, 2009; 174; 1154-59
21. Zoja C, Benigni A, Remuzzi G, Cellular responses to protein overload: Key event in renal disease progression: Curr Opin Nephrol Hypertens, 2004; 13; 31-37
22. Kamijo-Ikemori A, Sugaya T, Yasuda T, Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients: Diabetes Care, 2011; 34; 691-96
23. Gewin LS, Renal fibrosis: Primacy of the proximal tubule: Matrix Biol, 2018; 68–69; 248-62
24. Cho Y, Johnson DW, Vesey DA, Utility of urinary biomarkers in predicting loss of residual renal function: The Balanz trial: Perit Dial Int, 2015; 35; 159-71
25. Kamijo A, Sugaya T, Hikawa A, Urinary liver-type fatty acid binding protein as a useful biomarker in chronic kidney disease: Mol Cell Biochem, 2006; 284; 175-82
26. Cohen-Bucay A, Viswanathan G, Urinary markers of glomerular injury in diabetic nephropathy: Int J Nephrol, 2012; 2012; 146987
27. Wright JR, Duggal A, Thomas R, Clinicopathological correlation in biopsy-proven atherosclerotic nephropathy: Implications for renal functional outcome in atherosclerotic renovascular disease: Nephrol Dial Transplant, 2001; 16; 765-70
28. Nakamura T, Fujiwara N, Kawagoe Y, Effects of telmisartan and enalapril on renoprotection in patients with mild to moderate chronic kidney disease: Eur J Clin Invest, 2010; 40; 790-96
29. Nakamura T, Inoue T, Sugaya T, Renoprotective effect of telmisartan in patients with chronic kidney disease: Clin Exp Hypertens, 2008; 30; 662-72
Tables
 Table 1. Characteristics of patients on peritoneal dialysis.
Table 1. Characteristics of patients on peritoneal dialysis. Table 2. Comparison of clinical characteristics between RRF preservation and decline groups.
Table 2. Comparison of clinical characteristics between RRF preservation and decline groups. Table 3. Multiple logistic regression analysis of RRF decline in patients on PD.
Table 3. Multiple logistic regression analysis of RRF decline in patients on PD. Table 4. Simple and multiple regression analyses of the rate of RRF decline in patients on PD.
Table 4. Simple and multiple regression analyses of the rate of RRF decline in patients on PD. Table 1. Characteristics of patients on peritoneal dialysis.
Table 1. Characteristics of patients on peritoneal dialysis. Table 2. Comparison of clinical characteristics between RRF preservation and decline groups.
Table 2. Comparison of clinical characteristics between RRF preservation and decline groups. Table 3. Multiple logistic regression analysis of RRF decline in patients on PD.
Table 3. Multiple logistic regression analysis of RRF decline in patients on PD. Table 4. Simple and multiple regression analyses of the rate of RRF decline in patients on PD.
Table 4. Simple and multiple regression analyses of the rate of RRF decline in patients on PD. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952