24 March 2021: Database Analysis
Genome-Scale Analysis Identified , , and as Prognosis Markers of Overall Survival in Gastric Cancer
Zexing Shan1ACDE, Wentao Wang1B, Yilin Tong1B, Jianjun Zhang1B*DOI: 10.12659/MSM.929558
Med Sci Monit 2021; 27:e929558
Abstract
BACKGROUND: Gastric cancer is the most common gastrointestinal tumor, and the rates of recurrence and metastasis are high. Research results on molecular biomarkers used for prognosis of gastric cancer remain inconclusive. This study aimed to explore the gene expression module of gastric cancer and to determine potential prognostic biomarkers.
MATERIAL AND METHODS: Three microarray datasets (GSE13911, GSE79973, and GSE29272) from Gene Expression Omnibus (GEO), including 206 pairs of gastric tumors and adjacent normal samples, were used for analysis of differentially expressed genes (DEGs). The 3 microarray datasets yielded 144 genes associated with the progression and prognosis of gastric cancer. After this, a risk score model was developed for result validation using an independent dataset from The Cancer Genome Atlas.
RESULTS: The validation of the independent dataset showed significantly increased NID2, SPARC, and MFAP2 expression in gastric tumor tissues, which were associated with poor outcomes in gastric cancer patients. Moreover, the high risk score obtained was associated with poor overall survival (HR: 1.787; 1.069-2.986; P=0.027). Subgroup analyses revealed that these significant prognostic values were detected in patients aged <65.0 years, tumors in the antrum/distal colon, grade 3 tumors, or TNM-M0 stages of cancer.
CONCLUSIONS: The findings of this study show that NID2, SPARC, and MFAP2 are upregulated in gastric tumor tissues and are significantly associated with poor overall survival. Therefore, the predictive values of the risk score model employed for the prognosis of gastric cancer could be improved by using these 3 upregulated DEGs.
Keywords: Biological Markers, Models, Genetic, Biomarkers, Tumor, Calcium-Binding Proteins, Cell Adhesion Molecules, Databases, Genetic, Disease Progression, Gastric Mucosa, Gene Expression Profiling, Gene Regulatory Networks, Microarray Analysis, Osteonectin, RNA Splicing Factors
Background
Gastric cancer is the third leading cause of cancer-related deaths in the world, accounting for 8.2% of all cancer-related deaths [1,2]. Effective preventive and treatment strategies are required to improve the treatment and prognosis of gastric cancer, especially in Asian countries. Currently, the overall survival of gastric cancer has already been improved due to the diagnosis of the disease at an early stage and the timely application of adjuvant chemotherapy [3–5]. Although the advances in the multidisciplinary approaches and the combination treatment regimen, the prognosis of advanced gastric cancer remains dismal. Moreover, the heterogeneity of somatic or germline changes in patients are associated with the prognosis of gastric cancer.
Earlier studies have already identified the potential value of genetic and epigenetic alterations for gastric cancer prognosis. These alterations affect cycle regulation, cell adhesion, angiogenesis, and tumor carcinogenesis, having a significant prognostic role in the survival outcome in gastric cancer patients [6–9]. Moreover, investigations have already evaluated the gene expression profile of gastric cancer based on DNA microarray data, and explored the potential role of differentially expressed genes (DEGs) in the prognosis of gastric cancer [10–12]. However, the results of the above studies are limited due to their small sample sizes and the lack of validation datasets established in clinical practice. Hence, the use of the identified DEGs for prognosis of gastric cancer has been limited. Therefore, potential novel DEGs should be identified whose role in the overall survival in gastric cancer patients should be assessed.
The potential role of genes in the progression and prognosis of gastric cancer could be revealed through microarray analysis [13,14]. Three microarray data (GSE13911, GSE79973, and GSE29272) were integrated and 144 DEGs were identified. After the validation of DEGs in The Cancer Genome Atlas (TCGA), we noted that
Material and Methods
GASTRIC CANCER DATASETS:
The Gene Expression Omnibus (GEO,
DATA PREPROCESSING:
The raw probe-level data in this study were downloaded in CEL files, and the robust multi-array average algorithm RMA from the Affy package of R software was employed for processing the raw probe-level data [15]. The background correction, quantile normalization, and summarizing probe set values into 1 expression measure were processed for analysis of the data of gene expression. The annotations for the probe arrays were obtained from GEO, and the mean of the probe sets values was considered as a value of the expression when multiple probe sets were mapped to the same gene [16]. In our study, the log FC in datasets met the criteria for normal distribution.
STATISTICAL ANALYSIS:
The identified DEGs were evaluated using the LIMMA package, with bayesian adjusted t-statistics from the linear models for microarray data [17]. Genes with |log2 fold change (FC)| >1 and P<0.05 were regarded as DEGs between tumors and normal tissues. We constructed volcano plots and Venn diagrams using ggplot2, and Venn diagram packages of R software were used to visualize the identified DEGs.
The GO and KEGG pathway analyses of functional enrichment analysis for 144 common DEGs was conducted by using the online software Database for Annotation, Visualization, and Integrated Discovery (DAVID,
SPSS software (version 22.0, SPSS, Chicago, IL, USA) was used for statistical analysis. The risk score model consisted of gene expression, which could be validated in TCGA database. Next, the risk score model was constructed in TCGA-STAD, and the risk score of each individual patient was calculated. Moreover, the risk score was categorized into high and low, and the cutoff value was set to be the median of the risk score. The baseline characteristics between groups were compared using Kruskal-Wallis and chi-square tests based on the type of data. The propensity score analysis was used to adjust for imbalance in the baseline characteristics to avoid undue influences of confounding factors, which was analyzed using the MatchIt propensity score of R software, and the standardized mean difference for matching variables was defined as <20% between the groups. Kaplan-Meier and log-rank tests were employed for survival analysis. Subgroup analyses were also performed according to age, race, anatomic tumor site, grade, TNM-T, TNM-N, TNM-M, and stage.
Results
IDENTIFICATION OF DEGS BETWEEN GASTRIC TUMORS AND ADJACENT NORMAL SAMPLES:
GSE13911, GSE79973, and GSE29272 were employed as the discovery datasets for the identified DEGs expressed in gastric tumors and their adjacent normal tissues. These 3 datasets included 206 pairs of gastric tumors and their adjacent normal samples. The DEGs were explored to evaluate the association between gene expression alteration and gastric cancer progression. The details regarding the expression data from primary gastric tumors and adjacent normal samples are shown in Figure 1. A total number of 144 DEGs were detected for the intersecting part of the 3 sets, which were generally related to gastric samples and potentially associated with the progression and prognosis of gastric cancer (Figures 2, 3). Detailed information of the 144 DEGs established is presented in Table 1.
FUNCTIONAL ENRICHMENT ANALYSIS OF DEGS:
GO and KEGG pathway enrichment analyses were performed to investigate the biological roles of DEGs in gastric cancer progression, including cell cycle and cell adhesion. The enriched GO terms were mainly associated with the extracellular matrix of the cellular component, and the KEGG pathway analysis results showed that the most highly enriched pathway was ECM-receptor interaction. The results of the GO and KEGG pathway enrichment analyses are summarized and displayed in Tables 2 and 3.
VALIDATION OF DEGS IN AN INDEPENDENT DATABASE:
TCGA-STAD included 333 GC patients, who were regarded as a validation cohort, which was assessed to verify the expression of DEGs. The results indicated that
RISK SCORE AND OVERALL SURVIVAL FOR PATIENTS WITH GASTRIC CANCER:
The baseline characteristics of the high (n=166) and low (n=167) risk score groups are presented in Table 4. Significant differences were observed between groups in terms of race and tumor stages, whereas no significant differences were established for age, sex, anatomic tumor site, tumor grade, TNM-T, TNM-N, and TNM-M. Overall, we noted that a high risk score was obviously associated with poor overall survival (HR: 2.041; 95% CI: 1.272–3.274; P=0.003; Figure 4). Significant associations were observed mainly in the following patients: <65.0 years, with a tumor in the antrum/distal colon, with a grade 3 tumor, irrespective of the TNM-T stage, TNM-N2-3, TNM-M0, and stage III and IV (Table 2). After propensity score analysis, the higher risk scores were associated with poorer overall survival (HR: 1.787; 1.069–2.986; P=0.027; Figure 5). Subgroup analysis showed that high risk scores were associated with poor overall survival in patients age <65.0 years and if they had a tumor in the antrum/distal colon, grade 3 tumor, or TNM-M0 stages gastric cancer (Table 5).
Discussion
The gene expression modules at the genome-wide scale in gastric cancer were investigated in our study through integrating multiple gastric cancer transcriptome microarray datasets. Our findings provide information on alterations at the molecular level; we achieved higher robustness than that of data from a single dataset. We screened 144 DEGs in gastric tumors and adjacent normal samples and discovered that the expression levels of
The results of this study indicated that GC is involved in cell cycle, cell adhesion, and the extracellular matrix; these processes were found in patients with upregulated
We noted the expression of
Several limitations to this study should be acknowledged: (1) The interpretation of the results should be cautions due to the collection of data from different platforms; (2) Bioinformatics analysis was applied, whose findings should be verified in further research to clarify the mechanisms of the association between these genes and poor GC survival; (3) The range of the analyses was limited due to variations in the characteristics of the patients; and (4) The role of the expression of the studied 3 genes associated with other survival outcomes in patients with GC should be further explored, including the determination of progression-free survival.
Conclusions
In conclusion, the findings of this study suggest the upregulation of
Figures
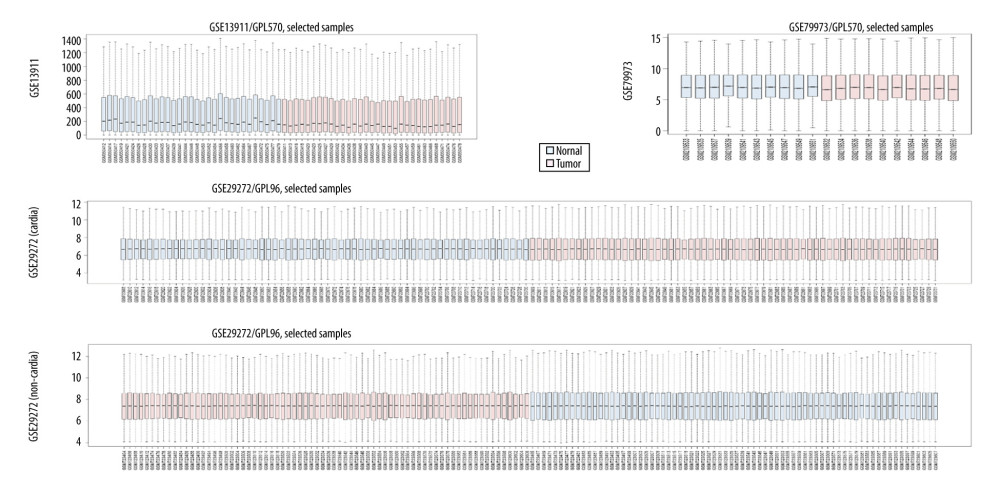 Figure 1. The details regarding the expression data from primary gastric tumors and adjacent normal samples in 4 subsets of 3 datasets.
Figure 1. The details regarding the expression data from primary gastric tumors and adjacent normal samples in 4 subsets of 3 datasets. 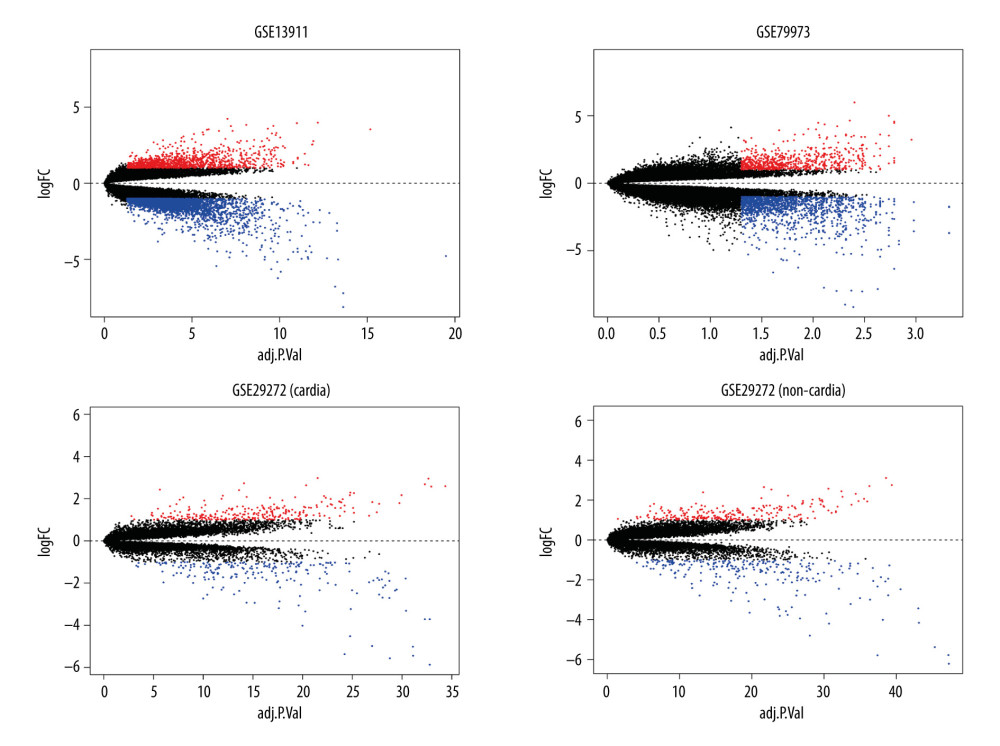 Figure 2. Identification of differentially expressed genes. Visualization of the identified differentially expressed genes was performed by volcano plots. Dots represent genes with color coding: red indicates upregulated, blue indicates downregulated, and black indicates genes that are not differentially expressed.
Figure 2. Identification of differentially expressed genes. Visualization of the identified differentially expressed genes was performed by volcano plots. Dots represent genes with color coding: red indicates upregulated, blue indicates downregulated, and black indicates genes that are not differentially expressed. 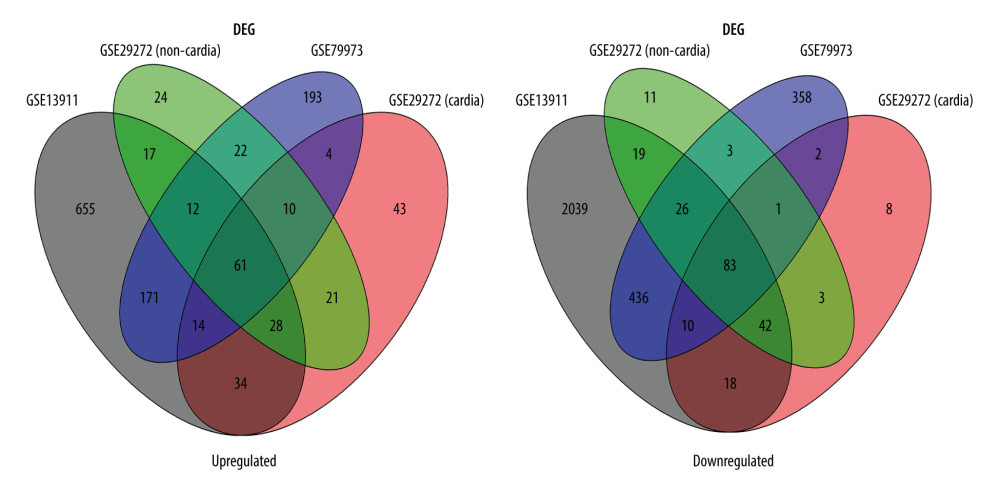 Figure 3. Venn diagram of the overlapping parts of the 4 subsets of 3 datasets of differentially expressed genes. Sixty-one genes were upregulated and 83 were downregulated.
Figure 3. Venn diagram of the overlapping parts of the 4 subsets of 3 datasets of differentially expressed genes. Sixty-one genes were upregulated and 83 were downregulated. 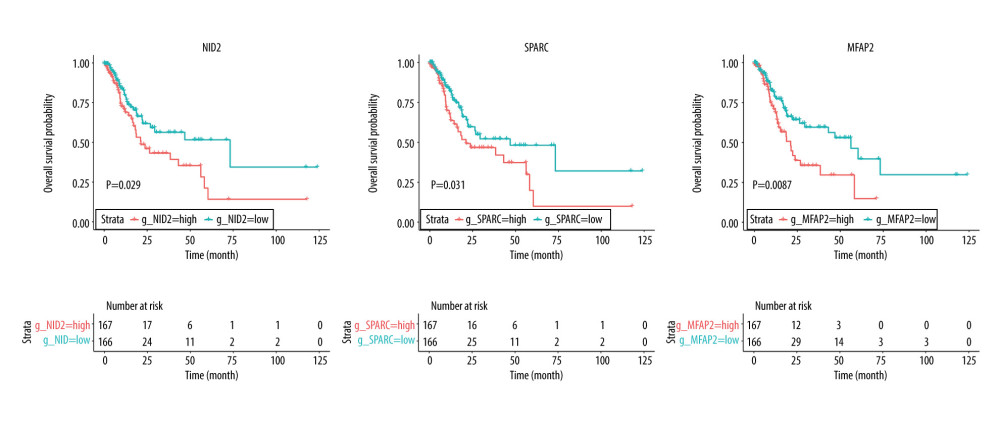 Figure 4. Overall survival according to the expression of NID2, SPARC, and MFAP2. Red line indicates high expression and blue line indicates low expression.
Figure 4. Overall survival according to the expression of NID2, SPARC, and MFAP2. Red line indicates high expression and blue line indicates low expression. 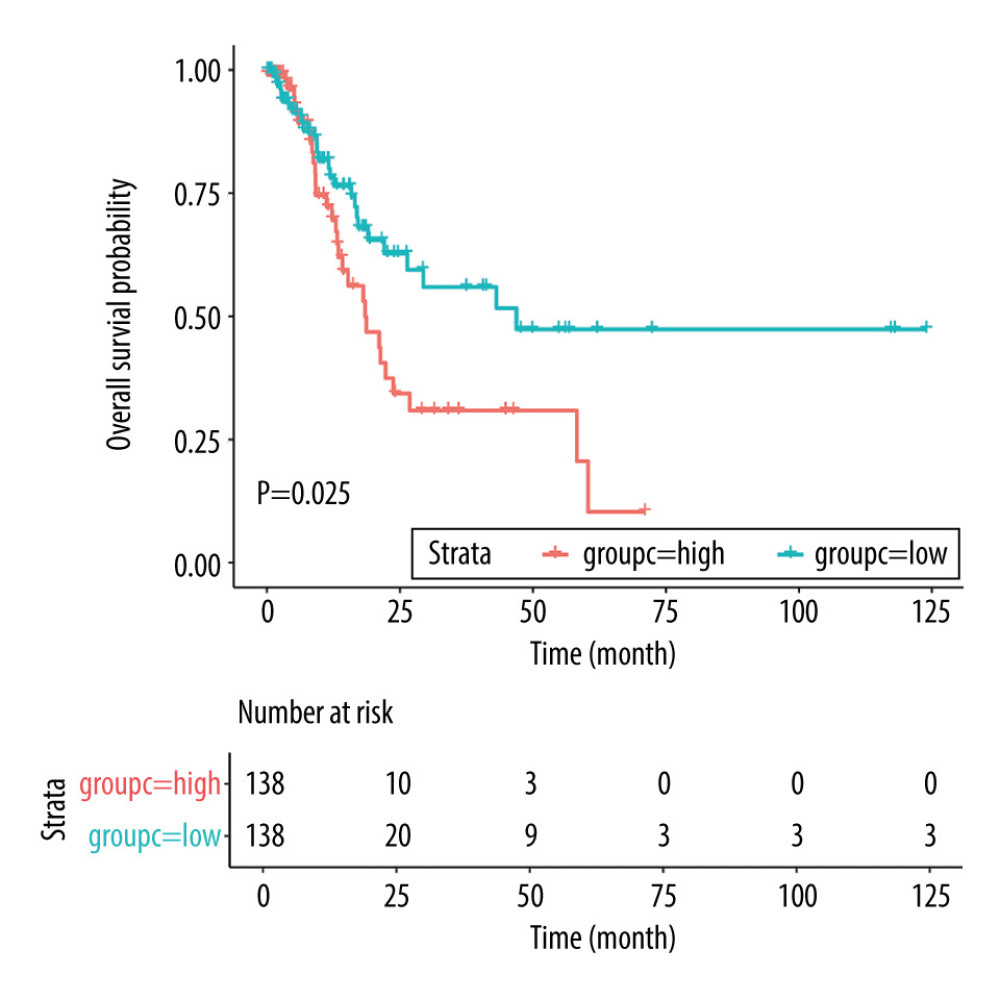 Figure 5. Overall survival according to the risk scores after propensity score analysis. Red line indicates high risk score and blue line indicates low risk score.
Figure 5. Overall survival according to the risk scores after propensity score analysis. Red line indicates high risk score and blue line indicates low risk score. Tables
Table 1. Common differentially expressed genes identified in gastric cancer.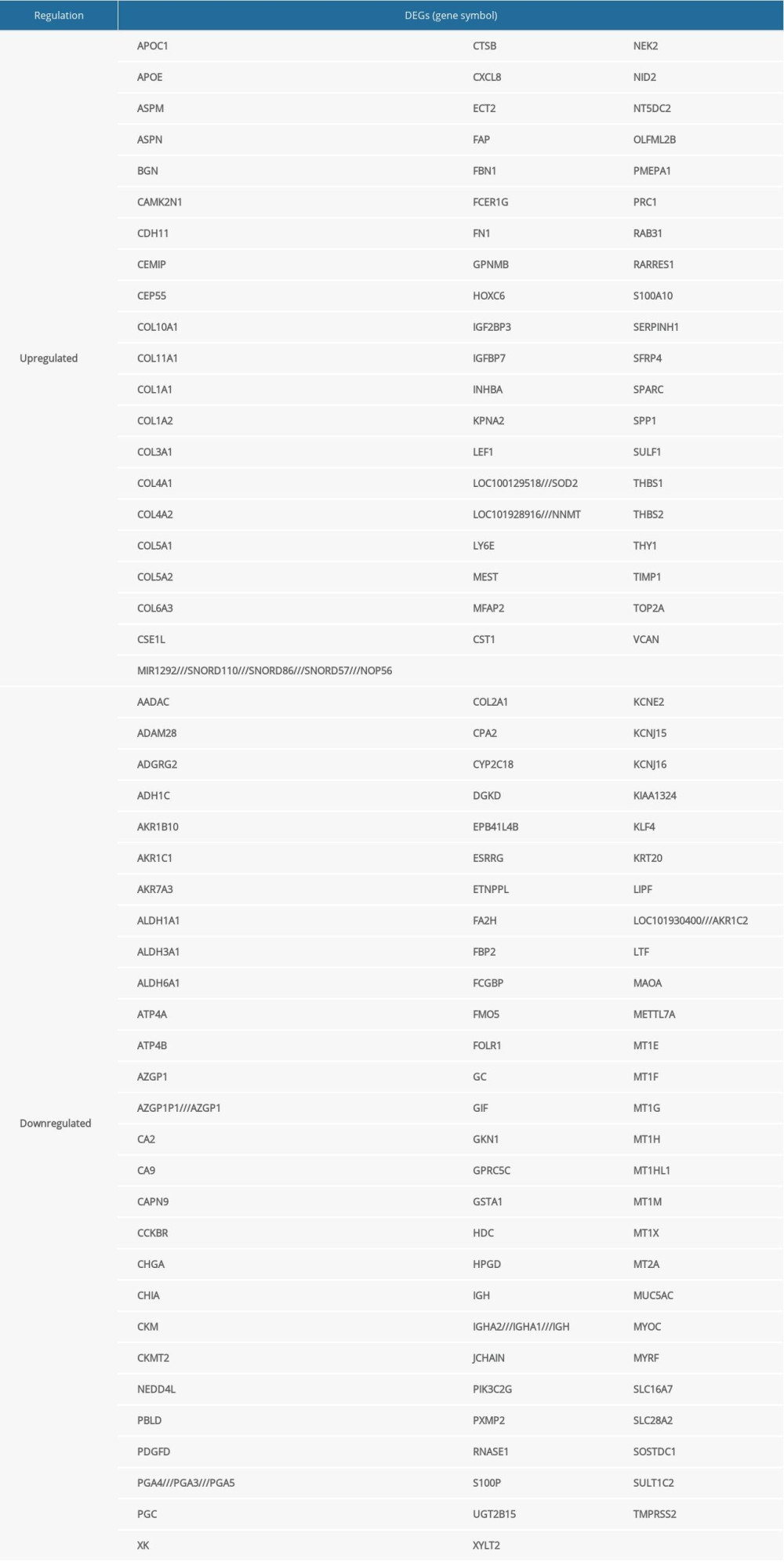 Table 2. GO analysis of the 144 differentially expressed genes.
Table 2. GO analysis of the 144 differentially expressed genes.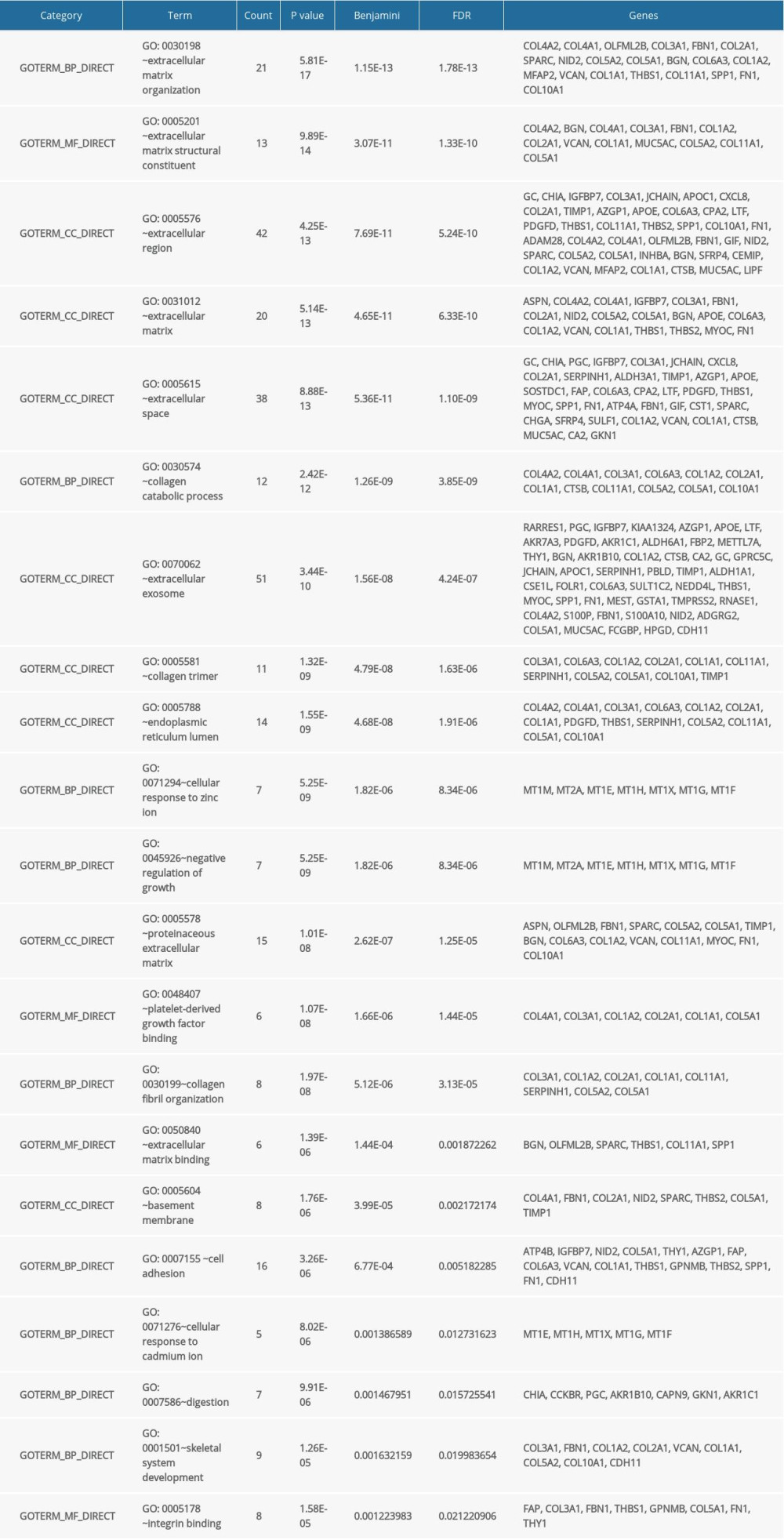 Table 3. KEGG pathway enrichment analysis of the 144 differentially expressed genes.
Table 3. KEGG pathway enrichment analysis of the 144 differentially expressed genes.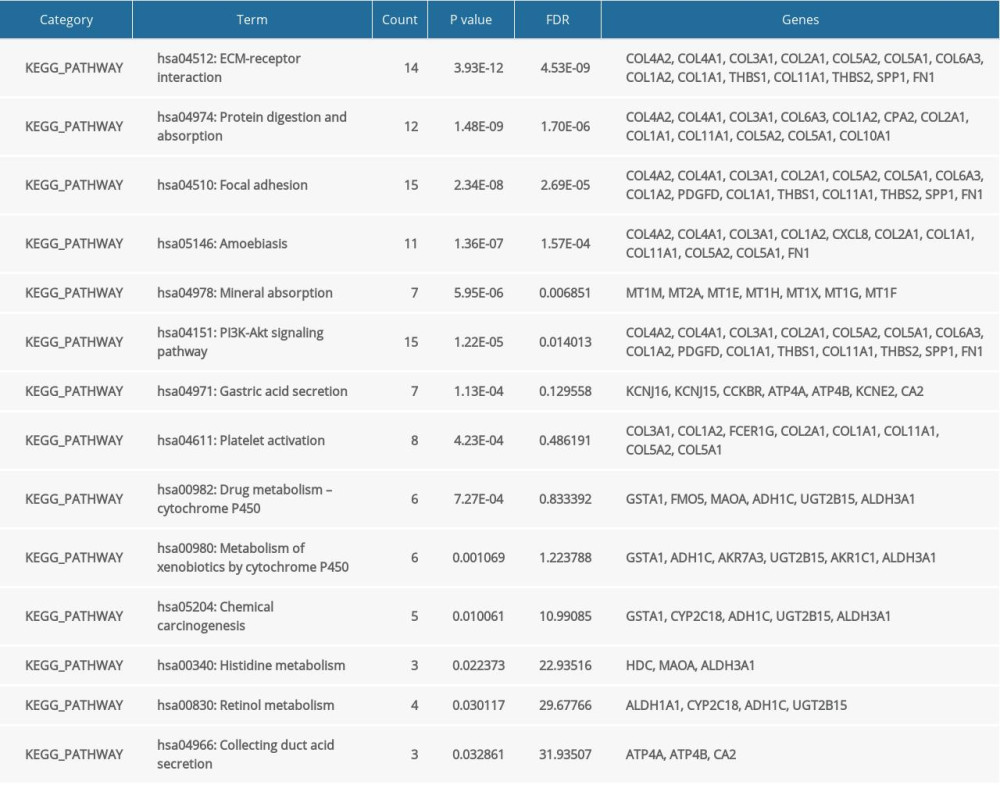 Table 4. Baseline characteristics of patients in high and low risk score groups.
Table 4. Baseline characteristics of patients in high and low risk score groups.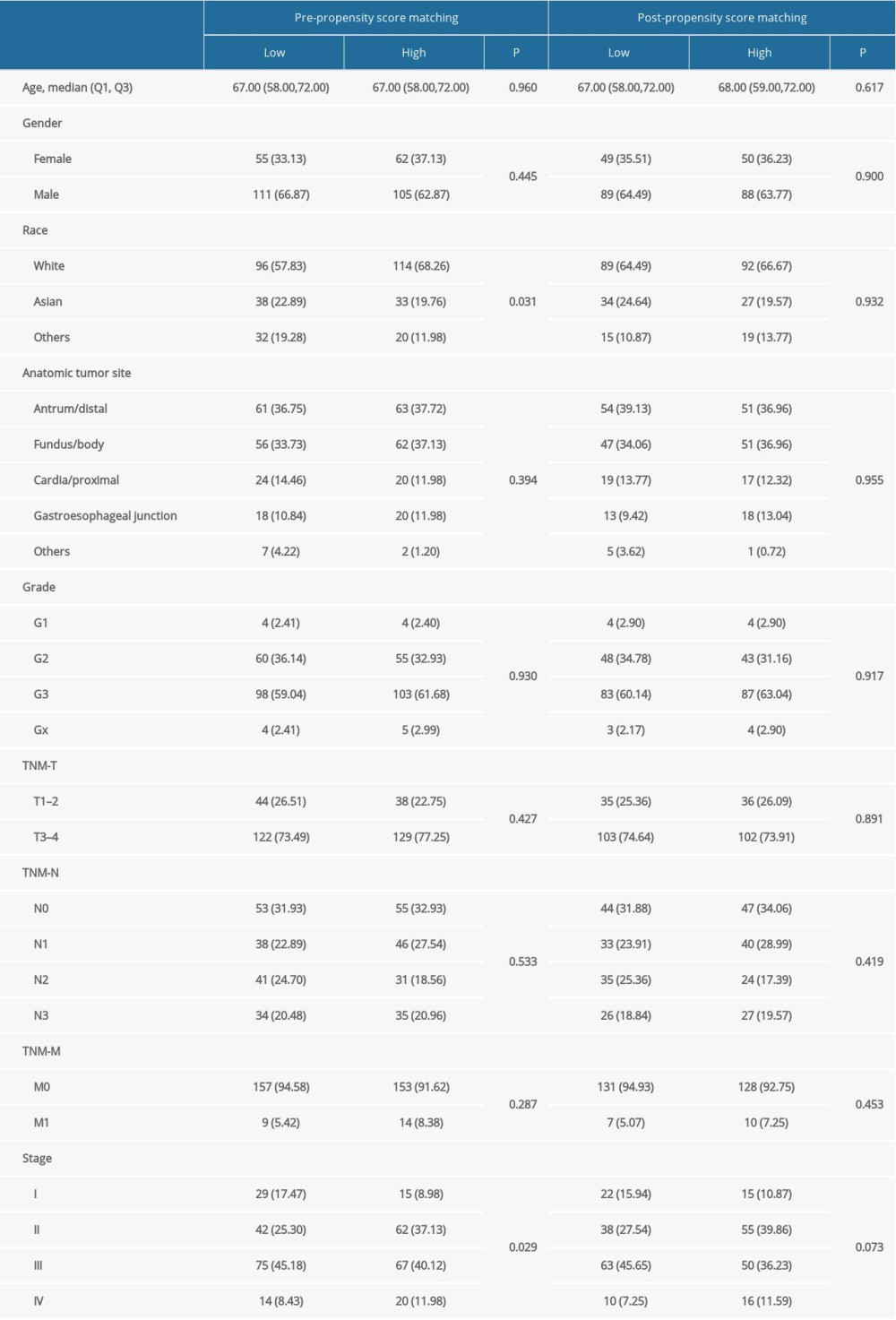 Table 5. Subgroup analyses for overall survival before and after propensity score analysis.
Table 5. Subgroup analyses for overall survival before and after propensity score analysis.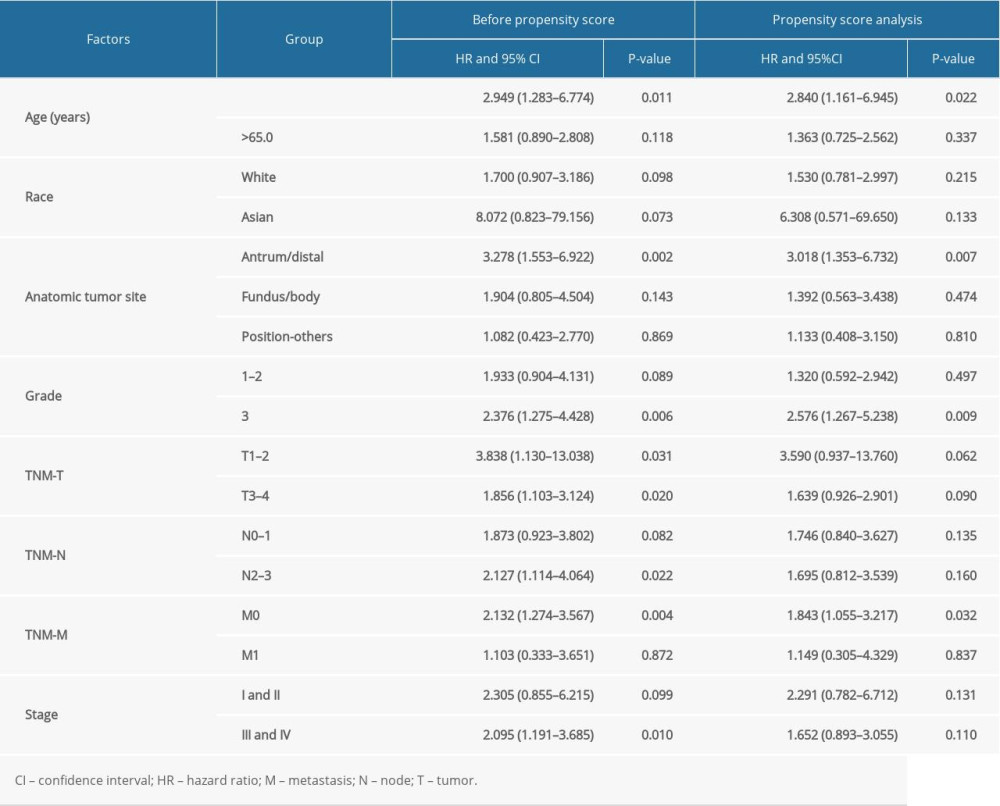
References
1. Ferlay J, Soerjomataram I, Ervik M: GLOBOCAN 2012 v10, Cancer Incidence and Mortality Worldwide:IARC CancerBase No. 11, Lyon, France, International Agency for Research on Cancer http://globocan.iarc.fr
2. Bray F, Ferlay J, Soerjomataram I, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2018; 68(6); 394-424
3. Information Committee of Korean Gastric Cancer Association, Korean Gastric Cancer Association Nationwide Survey on Gastric Cancer in 2014: J Gastric Cancer, 2016; 16(3); 131-40
4. Bang YJ, Kim YW, Yang HK, Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC) : A phase 3 open-label, randomised controlled trial: Lancet, 2012; 379(9813); 315-21
5. Sasako M, Sakuramoto S, Katai H, Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer: J Clin Oncol, 2011; 29(33); 4387-93
6. Akama Y, Yasui W, Yokozaki H, Frequent amplification of the cyclin E gene in human gastric carcinomas: Jpn J Cancer Res, 1995; 86(7); 617-21
7. Graziano F, Mandolesi A, Ruzzo A, Predictive and prognostic role of E-cadherin protein expression in patients with advanced gastric carcinomas treated with palliative chemotherapy: Tumour Biol, 2004; 25(3); 106-10
8. Tanigawa N, Amaya H, Matsumura M, Correlation between expression of vascular endothelial growth factor and tumor vascularity, and patient outcome in human gastric carcinoma: J Clin Oncol, 1997; 15(2); 826-32
9. Sanz-Ortega J, Steinberg SM, Moro E, Comparative study of tumor angiogenesis and immunohistochemistry for p53, c-ErbB2, c-myc and EGFr as prognostic factors in gastric cancer: Histol Histopathol, 2000; 15(2); 455-62
10. Cho JY, Lim JY, Cheong JH, Gene expression signature-based prognostic risk score in gastric cancer: Clin Cancer Res, 2011; 17(7); 1850-57
11. Chen CN, Lin JJ, Chen JJ, Gene expression profile predicts patient survival of gastric cancer after surgical resection: J Clin Oncol, 2005; 23(29); 7286-95
12. Wang X, Liu Y, Niu Z, Prognostic value of a 25-gene assay in patients with gastric cancer after curative resection: Sci Rep, 2017; 7(1); 7515
13. Chang W, Ma L, Lin L, Identification of novel hub genes associated with liver metastasis of gastric cancer: Int J Cancer, 2009; 125(12); 2844-53
14. Zhu T, Gao YF, Chen YX, Genome-scale analysis identifies GJB2 and ERO1LB as prognosis markers in patients with pancreatic cancer: Oncotarget, 2017; 8(13); 21281-89
15. Irizarry RA, Hobbs B, Collin F, Exploration, normalization, and summaries of high density oligonucleotide array probe level data: Biostatistics, 2003; 4(2); 249-64
16. Li W, Li K, Zhao L, Bioinformatics analysis reveals disturbance mechanism of MAPK signaling pathway and cell cycle in Glioblastoma multiforme: Gene, 2014; 547(2); 346-50
17. Diboun I, Wernisch L, Orengo CA, Microarray analysis after RNA amplification can detect pronounced differences in gene expression using limma: BMC Genomics, 2006; 7; 252
18. Liu JP, Liu D, Gu JF, Shikonin inhibits the cell viability, adhesion, invasion and migration of the human gastric cancer cell line MGC-803 via the Toll-like receptor 2/nuclear factor-kappa B pathway: J Pharm Pharmacol, 2015; 67(8); 1143-55
19. Gan L, Meng J, Xu M, Extracellular matrix protein 1 promotes cell metastasis and glucose metabolism by inducing integrin beta4/FAK/SOX2/HIF-1alpha signaling pathway in gastric cancer: Oncogene, 2018; 37(6); 744-55
20. Wu Q, Li X, Yang H, Extracellular matrix protein 1 is correlated to carcinogenesis and lymphatic metastasis of human gastric cancer: World J Surg Oncol, 2014; 12; 132
21. Wang J, Zhao Y, Xu H, Silencing NID2 by DNA hypermethylation promotes lung cancer: Pathol Oncol Res, 2020; 26(2); 801-11
22. Wu Q, Zhang B, Wang Z, Integrated bioinformatics analysis reveals novel key biomarkers and potential candidate small molecule drugs in gastric cancer: Pathol Res Pract, 2019; 215(5); 1038-48
23. van der Heijden AG, Mengual L, Ingelmo-Torres M, Urine cell-based DNA methylation classifier for monitoring bladder cancer: Clin Epigenetics, 2018; 10; 71
24. Chai AWY, Cheung AKL, Dai W, Elevated levels of serum nidogen-2 in esophageal squamous cell carcinoma: Cancer Biomark, 2018; 21(3); 583-90
25. Torky HA, Sherif A, Abo-Louz A, Evaluation of serum Nidogen-2 as a screening and diagnostic tool for ovarian cancer: Gynecol Obstet Invest, 2018; 83(5); 461-65
26. Liao P, Li W, Liu R, Genome-scale analysis identifies SERPINE1 and SPARC as diagnostic and prognostic biomarkers in gastric cancer: Onco Targets Ther, 2018; 11; 6969-80
27. Li Z, Li AD, Xu L, SPARC expression in gastric cancer predicts poor prognosis: Results from a clinical cohort, pooled analysis and GSEA assay: Oncotarget, 2016; 7(43); 70211-22
28. Wang Z, Hao B, Yang Y, Prognostic role of SPARC expression in gastric cancer: A meta-analysis: Arch Med Sci, 2014; 10(5); 863-69
29. Funk SE, Sage EH, The Ca2(+)-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells: Proc Natl Acad Sci USA, 1991; 88(7); 2648-52
30. Lane TF, Sage EH, The biology of SPARC, a protein that modulates cell-matrix interactions: FASEB J, 1994; 8(2); 163-73
31. Mecham RP, Gibson MA, The microfibril-associated glycoproteins (MAGPs) and the microfibrillar niche: Matrix Biol, 2015; 47; 13-33
32. Zaravinos A, Kanellou P, Lambrou GI, Gene set enrichment analysis of the NF-kappaB/Snail/YY1/RKIP circuitry in multiple myeloma: Tumour Biol, 2014; 35(5); 4987-5005
33. Silveira NJ, Varuzza L, Machado-Lima A, Searching for molecular markers in head and neck squamous cell carcinomas (HNSCC) by statistical and bioinformatic analysis of larynx-derived SAGE libraries: BMC Med Genomics, 2008; 1; 56
Figures
 Figure 1. The details regarding the expression data from primary gastric tumors and adjacent normal samples in 4 subsets of 3 datasets.
Figure 1. The details regarding the expression data from primary gastric tumors and adjacent normal samples in 4 subsets of 3 datasets. Figure 2. Identification of differentially expressed genes. Visualization of the identified differentially expressed genes was performed by volcano plots. Dots represent genes with color coding: red indicates upregulated, blue indicates downregulated, and black indicates genes that are not differentially expressed.
Figure 2. Identification of differentially expressed genes. Visualization of the identified differentially expressed genes was performed by volcano plots. Dots represent genes with color coding: red indicates upregulated, blue indicates downregulated, and black indicates genes that are not differentially expressed. Figure 3. Venn diagram of the overlapping parts of the 4 subsets of 3 datasets of differentially expressed genes. Sixty-one genes were upregulated and 83 were downregulated.
Figure 3. Venn diagram of the overlapping parts of the 4 subsets of 3 datasets of differentially expressed genes. Sixty-one genes were upregulated and 83 were downregulated. Figure 4. Overall survival according to the expression of NID2, SPARC, and MFAP2. Red line indicates high expression and blue line indicates low expression.
Figure 4. Overall survival according to the expression of NID2, SPARC, and MFAP2. Red line indicates high expression and blue line indicates low expression. Figure 5. Overall survival according to the risk scores after propensity score analysis. Red line indicates high risk score and blue line indicates low risk score.
Figure 5. Overall survival according to the risk scores after propensity score analysis. Red line indicates high risk score and blue line indicates low risk score. Tables
 Table 1. Common differentially expressed genes identified in gastric cancer.
Table 1. Common differentially expressed genes identified in gastric cancer. Table 2. GO analysis of the 144 differentially expressed genes.
Table 2. GO analysis of the 144 differentially expressed genes. Table 3. KEGG pathway enrichment analysis of the 144 differentially expressed genes.
Table 3. KEGG pathway enrichment analysis of the 144 differentially expressed genes. Table 4. Baseline characteristics of patients in high and low risk score groups.
Table 4. Baseline characteristics of patients in high and low risk score groups. Table 5. Subgroup analyses for overall survival before and after propensity score analysis.
Table 5. Subgroup analyses for overall survival before and after propensity score analysis. Table 1. Common differentially expressed genes identified in gastric cancer.
Table 1. Common differentially expressed genes identified in gastric cancer. Table 2. GO analysis of the 144 differentially expressed genes.
Table 2. GO analysis of the 144 differentially expressed genes. Table 3. KEGG pathway enrichment analysis of the 144 differentially expressed genes.
Table 3. KEGG pathway enrichment analysis of the 144 differentially expressed genes. Table 4. Baseline characteristics of patients in high and low risk score groups.
Table 4. Baseline characteristics of patients in high and low risk score groups. Table 5. Subgroup analyses for overall survival before and after propensity score analysis.
Table 5. Subgroup analyses for overall survival before and after propensity score analysis. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








