12 April 2022: Clinical Research
Development and Validation of a Nomogram for Predicting Postoperative Distant Metastasis in Patients with Cervical Cancer
Weihong Zeng1ABCDE, Lishan Huang1BCD, Haihong Lin1BCD, Ru Pan1BCD, Haochang Liu1BCD, Jizhong Wen1BCD, Ye Liang1BCD, Haikun Yang1AE*DOI: 10.12659/MSM.933379
Med Sci Monit 2022; 28:e933379
Abstract
BACKGROUND: Cervical cancer is the fourth most commonly diagnosed malignant neoplasm among women worldwide. Despite improvements in treatment, the rate of postoperative metastasis remains a problem. Nomograms have been used to predict risk of tumor metastasis. We designed a nomogram to predict postoperative distant metastasis among cervical cancer patients, based on the SEER database, and estimated the performance of the nomogram by internal and external validations.
MATERIAL AND METHODS: We included 6421 participants and divided them into training (n=4495) and testing (n=1926) sets. Multivariate logistic regression was used to explore predictors. The nomogram’s predictive value was assessed by internal (testing set) and external (561 Chinese patients) validations. The receiver operating characteristic curve (ROC) was plotted, and the area under the curve (AUC) value was calculated to evaluate the nomogram’s discrimination. The nomogram’s calibration was assessed via the Hosmer-Lemeshow test and calibration curve.
RESULTS: Histologic type, T stage, treatment, tumor size, and positive lymph node were identified as independent predictors of postoperative distant metastasis in surgical patients (P<0.05). The developed nomogram had an AUC of 0.866 (95% CI: 0.844 to 0.888). The AUC and the chi-square for the Hosmer-Lemeshow test of the nomogram were 0.847 (95% CI: 0.807 to 0.888) and 11.292, respectively, (P>0.05) in the internal validation, and were 0.626 (95% CI: 0.548 to 0.704) and 316.53, respectively, (P<0.05) in the external validation.
CONCLUSIONS: Our nomogram showed a good predictive performance for postoperative distant metastasis in cervical cancer patients based on the SEER database. It remains to be determined if it is applicable to other populations.
Keywords: database, nomograms, Validation study, Area Under Curve, Female, Humans, ROC Curve
Background
Cervical cancer is the fourth most commonly diagnosed malignant neoplasm among women worldwide [1]. It was estimated that 569 847 new cervical cancer cases and 311 365 deaths occur each year [2]. Despite significant improvements in the treatment of cervical cancer by surgical resection, the rate of postoperative distant metastasis remains an intractable problem, which has been proved to be associated with cervical cancer-related death [3,4]. Therefore, it is vital for physicians to be able to predict postoperative distant metastasis to improve the prognosis of cervical cancer patients.
Some predictors, such as pelvic lymph node metastasis [5], histologic type [6,7], and tumor size [8,9], have been revealed in previous studies to be associated with high risk of recurrence in patients with cervical cancer after surgical therapy. Several postoperative nomograms have been developed to predict the risk of recurrence in early-stage cervical cancer [10–13]. Moreover, Lee et al built a scoring system based on histologic type, the number of positive nodes, and surgical staging, which was used to assess the risk of distant recurrence in cervical cancer patients after radical surgery [14]. Although some investigations have focused on the predictive factors of distant metastasis in cervical cancer patients [15–17], scant evidence is available on models to predict the risk of distant metastasis after surgery in patients with cervical cancer. Je et al proposed a nomogram based on 1069 Korean patients with uterine cervical carcinoma undergoing postoperative radiotherapy to predict distant metastasis risks in 2014 [18], which was successfully validated among 109 cervical cancer patients from 3 branch hospitals of the Korea University Medical Center in 2017 [19]. Nomograms have been used to predict the possibility of tumor metastasis, and can visualize complex regression equations to make predictive results more intuitive and convenient for clinicians to use [20–22]. At present, no nomograms are available for other countries except Korea to predict the postoperative distant metastasis of cervical cancer.
In the present study, we constructed a clinical nomogram to predict postoperative distant metastasis among cervical cancer patients based on the Surveillance, Epidemiology, and End Results (SEER) database, and further estimated the predictive performance of the nomogram by internal and external validations.
Material and Methods
DATA SOURCES AND STUDY DESIGN:
The SEER database records information about demographics, primary tumor site, tumor morphology, stage at diagnosis, the first course of treatment, and vital status after follow-up for the US population. In this retrospective cohort study, data on cervical cancer cases were obtained from the SEER 18 Regs Custom Data (with additional treatment fields) of the National Cancer Institute (
DATA EXTRACTION:
The following data were extracted from the SEER database: age at diagnosis, histologic type (squamous cell carcinoma, adenocarcinoma, adenosquamous carcinoma, and others), tumor grade, treatment (surgery, surgery+chemotherapy, surgery+radiotherapy, and surgery+radiotherapy+chemotherapy), T stage, N stage, tumor size, first malignant primary, regional nodes, regional nodes positive, and CS metastasis at DX. In this study, the outcome was postoperative distant metastasis, which was confirmed by the “CS mets at DX”, with “Yes” suggesting the occurrence of postoperative distant metastasis.
DEVELOPMENT AND VALIDATION OF THE NOMOGRAM:
To develop and validate the nomogram, all the enrolled patients were randomly divided into 2 sets by random number generation: training (n=4495) and testing (n=1926) sets. For the training set, factors that were statistically different between non-metastasis and metastasis groups through difference analysis were included in multivariate stepwise logistic regression analysis (rms package) to identify independent predictors for postoperative distant metastasis in patients with cervical cancer. Based on the identified predictors, the nomogram was constructed to predict postoperative distant metastasis for cervical cancer.
The testing set was utilized for interval validation of the nomogram, and external validation was conducted using clinical data from 561 Chinese cervical cancer patients who developed distant metastasis during postoperative follow-ups from the Meizhou People’s Hospital between 3 February 2015 and 1 July 2020, which was approved by the Ethics Committee of the Meizhou People’s Hospital (Huangtang Hospital), Meizhou Academy of Medical Sciences (2019-C-47). To further assess the applicability of the nomogram in the Chinese population, the nomogram’s predictive ability was evaluated in subgroups stratified by age, histologic type, T stage, treatment, tumor size, and lymph node positivity. The receiver operating characteristic curve (ROC) was plotted, and the corresponding area under the curve (AUC) value (pROC package) was applied to evaluate the discrimination ability of the nomogram. Meanwhile, McNemar’s test, Hosmer-Lemeshow test (ResourceSelection package), and calibration curve were used to assess the calibration of the nomogram.
STATISTICAL ANALYSIS:
Each statistical test was two-sided, and
Results
BASELINE CHARACTERISTICS:
A total of 6421 patients were enrolled in this study, with 4495 patients in the training cohort and 1926 in the testing cohort. In the training cohort the average age was 47.15±12.42 years, and there were 2543 (56.57%) patients with squamous cell carcinoma, 783 (17.42%) patients with adenocarcinoma, 226 (5.03%) patients with adenosquamous carcinoma, and 943 (20.98%) patients with other histopathologic cell types. The tumor grades were: I (2033/45.23%), II (1486/33.06%), III (115/2.56%), and IV (861/19.15%). According to “SEER RESEARCH PLUS DATA DESCRIPTION CASES DIAGNOSED IN 1975–2018”, cervical cancers have 4 tumor grades: Grade I (well differentiated), Grade II (moderately differentiated), Grade III (poorly differentiated), and Grade IV (undifferentiated). Of the 4495 patients in the training cohort, 2454 (54.59%) were treated with surgery only, 164 (3.65%) with surgery and chemotherapy, 482 (10.72%) with surgery and radiotherapy, and 1395 (31.03%) with surgery, radiotherapy, and chemotherapy. There were 190 (4.23%) patients with postoperative metastasis and 4305 (95.77%) without postoperative metastasis (Figure 1, Table 1).
IDENTIFICATION OF PREDICTIVE FACTORS BASED ON THE TRAINING SET:
Age, histologic type, tumor grade, number of lymph nodes, T stage, N stage, treatment, tumor size (≥4 cm), first malignant primary, and lymph node positivity were significantly different between patients with and without postoperative distant metastasis in the training set according to univariate analysis (P<0.05) (Table 2). However, only histologic type, T stage, treatment, tumor size, and positive lymph node were identified to be significantly associated with postoperative metastasis in multivariate analysis (P<0.05) (Figure 2).
NOMOGRAM CONSTRUCTION AND EVALUATION:
Based on the predictive factors, including histologic type, T stage, treatment, tumor size, and lymph node positivity, the prediction model was constructed: Y=−5.761+0.746×histologic type (adenosquamous carcinoma)+0.879×histologic type (other)+0.930× T stage (T2)+1.876× T stage (T3)+1.923× T stage (T4)+2.378× treatment (surgery and chemotherapy)+0.759× treatment (surgery and radiotherapy)+0.723× treatment (surgery, radiotherapy, and chemotherapy)+0.769× tumor size (≥4 cm)+1.272× lymph node positivity (yes). The nomogram prediction of postoperative distant metastasis was presented (Figure 3), with an AUC of 0.866 (95% CI: 0.844 to 0.888) (Figure 4). These results showed that the nomogram had good predictive ability in the training cohort.
NOMOGRAM VALIDATION:
Internal and external validations were conducted to assess the predictive value of the nomogram. In the internal validation, the AUC for the predictive nomogram was 0.847 (95% CI: 0.807 to 0.888) (Figure 5A), and the chi-squares of the McNemar’s and Hosmer-Lemeshow tests were 0.039 (P>0.05) and 11.292 (P>0.05), respectively (Table 3). Meanwhile, a calibration curve for the predictive nomogram was drawn in the internal validation cohort, indicating good calibration (Figure 5B). The results of internal validation indicated that the nomogram had good discrimination and calibration abilities. In the external validation, the AUC for the predictive nomogram was 0.626 (95% CI: 0.548 to 0.704) (Figure 6A), and the chi-squares of the McNemar’s and Hosmer-Lemeshow tests were 111.484 (P<0.05) and 316.53 (P<0.05), respectively (Table 4). Meanwhile, a calibration curve for the predictive nomogram was drawn in the external validation cohort, indicating poor calibration (Figure 6B). These findings of the external validation suggested that the discrimination and calibration abilities of the nomogram were poor in the Chinese cohort. To further assess the applicability of the nomogram in the Chinese population, the nomogram’s predictive ability was evaluated in subgroups stratified by age, histologic type, T stage, treatment, tumor size, and lymph node positivity. We found that the AUC of the nomogram for cervical cancer patients with adenocarcinoma was 0.811 (95% CI: 0.618 to 1.000) (Table 5). These results implied that the nomogram exhibited good performance in predicting postoperative distant metastasis among cervical cancer patients with adenocarcinoma.
Discussion
Early diagnosis and surgical treatment have been advocated for cervical cancer patients. However, the occurrence of postoperative distant metastasis remains a complex problem, and nomograms to accurately estimate postoperative distant metastasis for cervical cancer await more exploration. The present study developed and validated a novel prediction tool for the risk of postoperative distant metastasis in cervical cancer patients. The selected predictive factors included histologic type, T stage, treatment, tumor size, and positive lymph node. Based on these predictors, a nomogram for predicting the risk of postoperative distant metastasis in cervical cancer patients was established, with an AUC of 0.866. In addition, the AUC of the nomogram was 0.847, with the chi-square for Hosmer-Lemeshow test of 11.292 (
From the perspective of treatments, adjuvant treatment (including radiotherapy, chemotherapy, or radiotherapy combined with chemotherapy) would increase the risk of postoperative distant metastasis in cervical cancer patients. The chemotherapy agent is mainly used to act against tumor cells to shrink lesions. However, it can not only act on tumor cells but can also inhibit normal cells in other parts of the body to varying degrees, which causes damage to the body and induces a series of host reactions, forming a more suitable microenvironment for metastasis of malignant tumors. A previous review underscores the paradoxical pro-metastatic impacts of chemotherapy via remodeling of the primary tumor and generation of a favorable metastasis-promoting niche [23]. In addition, M2-type macrophages not only facilitate tumor growth and angiogenesis [24–26], but also promote tumor invasion and metastasis [27–30]. It has been reported that some chemotherapy drugs can induce monocytes to differentiate into M2-type macrophages by promoting the secretion of interleukin-6 and prostaglandin-2 in cervical cancer cells [31]. Ionizing radiation (IR) has been shown to promote tumor metastasis [32], and stimulate pro-metastatic cellular activities [33]. IR paradoxically facilitates metastasis and invasion of cancer cells via inducing epithelial-mesenchymal transition (EMT), hindering cancer management [34]. High-dose IR can induce endothelial cell dysfunction, but low-dose IR can promote the formation of capillaries by increasing the survival of endothelial cells induced by activation of the Akt-pathway, thus promoting tumor metastasis [35]. Administering vascular endothelial growth factor (VEGF) receptor-tyrosine kinase inhibitors immediately before IR exposure can prevent low-dose IR from promoting tumor growth and metastasis [36]. Beyond that, patients receiving surgery and chemotherapy or radiotherapy should have a regular follow-up after surgery.
Tumor size, T stage, and histologic type were independent predictors of postoperative distant metastasis in cervical cancer patients. A previous study indicated that a tumor size larger than 4 cm increased the risk of recurrence in cervical cancer patients [37]. Our results indicated that tumor size ≥4 cm was a predictor of increased distant metastasis in cervical cancer patients after surgery. Generally, a larger tumor with an adequate blood supply has longer growth time, increasing the possibility of lymph node metastasis, local infiltration, and distant metastasis, and the tumor can be prone to recurrence and distant metastasis after surgery. It has been proven that with the increase of tumor size, the number of tumor cells in the peripheral blood increases [38]. A study revealed that the number of tumor cells in bone marrow was positively correlated with tumor size in patients with cervical cancer [39]. From the perspective of the patients’ condition, positive lymph node status was determined as an independent predictor of distant metastasis in cervical cancer patients after surgery. Lymph node metastasis has been confirmed to be a risk factor in cervical cancer patients who underwent surgery [40,41], which confirmed our result that lymph node positivity was a predictor of the increased risk of distant metastasis.
Although previous studies have developed several prediction models for the distant metastasis of cervical cancer, our research supplements these studies. Wang et al incorporated log of odds between the number of positive lymph nodes and the number of negative lymph nodes (LODDS) in establishing a prognostic nomogram for cervical cancer patients after surgery [42]. However, they focused on overall survival of patients with cervical cancer. In the present study, we specifically constructed a nomogram to predict postoperative distant metastasis of cervical cancer, with AUCs of 0.866 and 0.847 in the training and validation cohorts, respectively. Future studies can take LODDS into consideration for predicting postoperative distant metastasis among cervical cancer patients. A model to predict distant metastasis in cervical cancer patients was developed by Liu et al [43], and the differences between their model and our model are as follows. On the one hand, regarding subjects, cervical cancer patients treated with definitive radiotherapy were included in the study of Liu et al
A strength of the present study is its relatively large sample size. Moreover, external validation of the predictive nomogram was carried out. However, our research indicated that the nomogram might not be applied to the Chinese population, possibly due to differences between Chinese and US populations. Our study also has several limitations. First, this was a retrospective study based on the SEER database. Second, the prediction model was validated in only 1 hospital. Patients from different institutions or ethnicities are needed to conduct external validations. Third, owing to the limited data collected, the effects of different surgical treatments and chemotherapy drugs on postoperative distant metastasis could not be evaluated, and further studies are required to explore the relationships between them.
Conclusions
Our nomogram had good predictive performance for postoperative distant metastasis in patients with cervical cancer based on 6421 participants from the SEER database, which may serve as a reference for clinicians to identify cervical cancer patients with a high risk of postoperative distant metastasis early to provide individualized therapy. However, whether the nomogram is applicable to other populations remains to be determined.
Supplementary Material
The diagnosis of cervical cancer was confirmed through the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3), primary site codes C53.0, C53.1, C53.8, C53.9, combined with histology codes 8000, 8001, 8010, 8012, 8013, 8015, 8020, 8022, 8033, 8041, 8042, 8045, 8046, 8050, 8051, 8052, 8070, 8071, 8072, 8073, 8074, 8075, 8076, 8082, 8083, 8084, 8094, 8098, 8120, 8123, 8130, 8140, 8144, 8200, 8210, 8240, 8244, 8246, 8255, 8260, 8261, 8262, 8263, 8310, 8313, 8323, 8380, 8382, 8384, 8410, 8441, 8460, 8461, 8480, 8481, 8482, 8490, 8500, 8542, 8560, 8570, 8574, 8720, 8800, 8801, 8802, 8805, 8890, 8896, 8900, 8910, 8912, 8920, 8931, 8933, 8935, 8950, 8963, 8980, 9044, 9080, 9100, 9110, 9473, 9581.
Figures
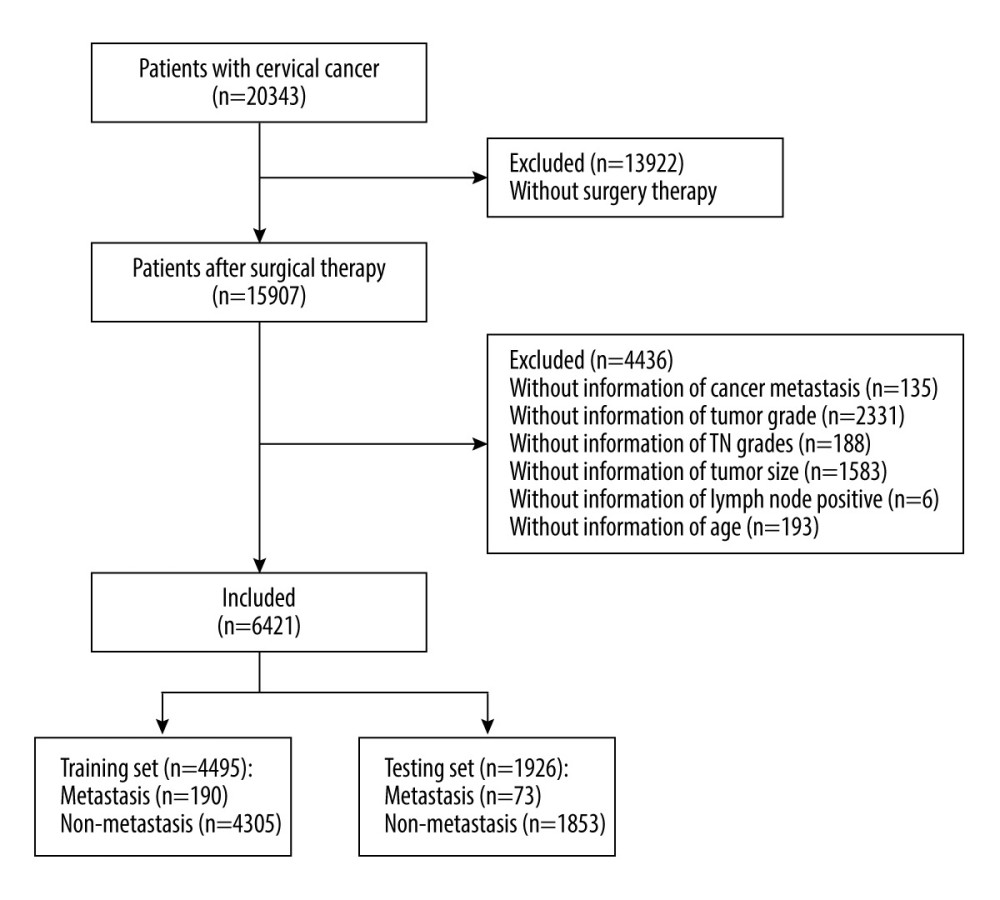 Figure 1. Flow chart for screening included patients with cervical cancer. Draw.io (version 12.6.5.330, JGraph Ltd.) was used for figure creation.
Figure 1. Flow chart for screening included patients with cervical cancer. Draw.io (version 12.6.5.330, JGraph Ltd.) was used for figure creation. 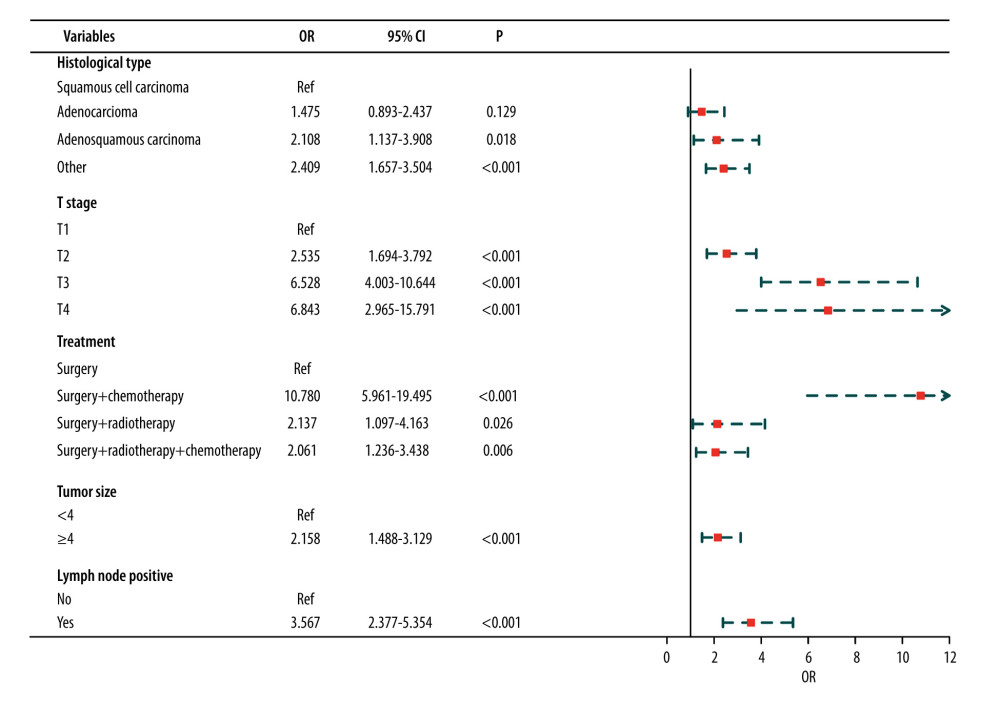 Figure 2. Results of multivariate analysis. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation.
Figure 2. Results of multivariate analysis. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation. 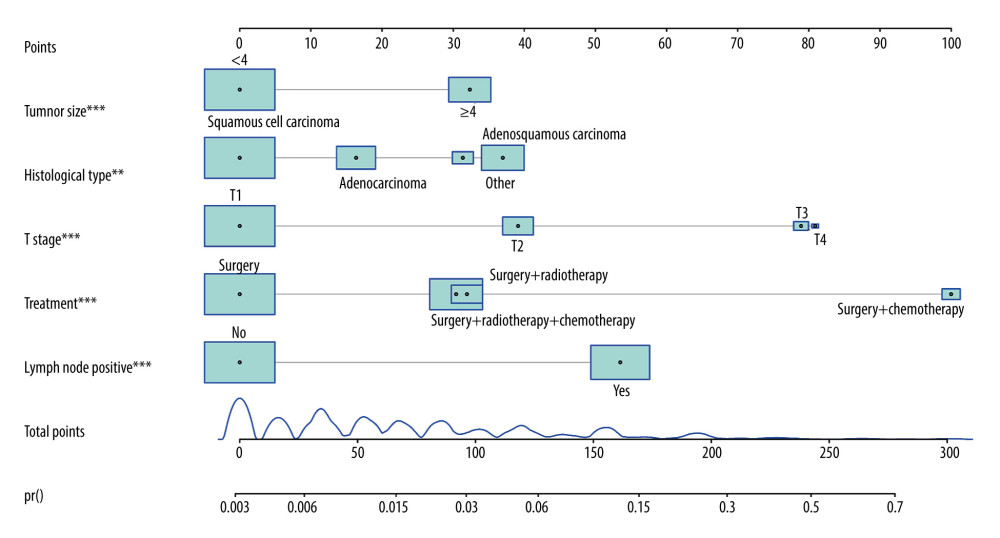 Figure 3. Nomogram prediction of postoperative metastasis. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation.
Figure 3. Nomogram prediction of postoperative metastasis. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation. 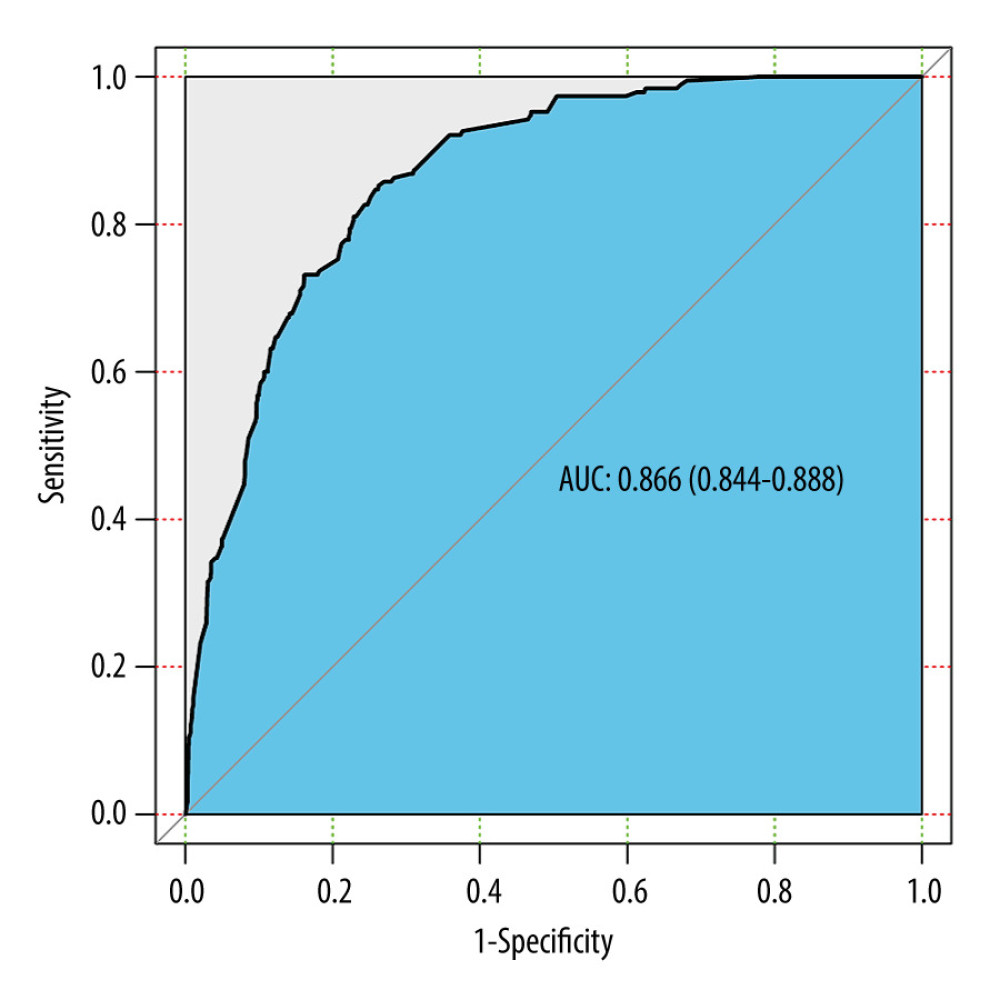 Figure 4. ROC curve of the predictive nomogram. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation.
Figure 4. ROC curve of the predictive nomogram. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation. 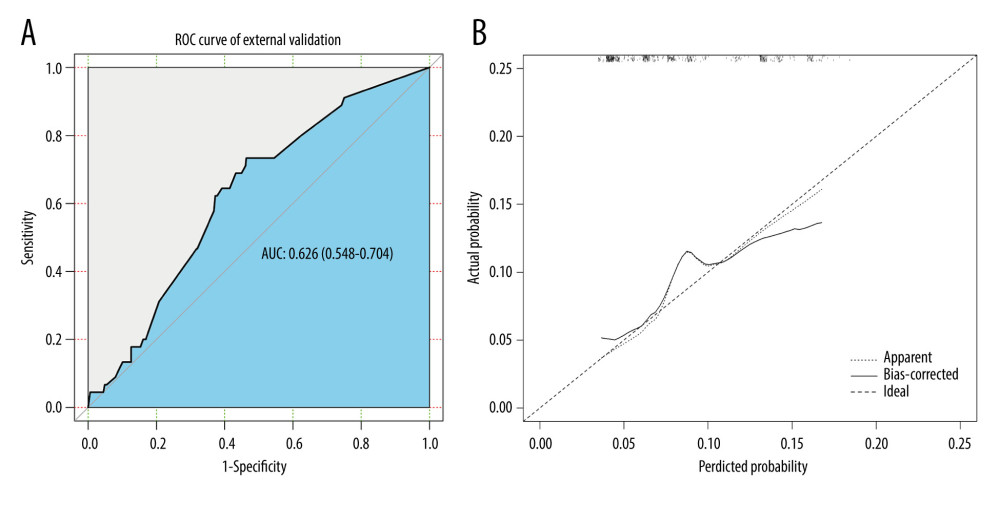 Figure 5. The (A) ROC curves and (B) calibration curve of the internal validation set. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation.
Figure 5. The (A) ROC curves and (B) calibration curve of the internal validation set. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation. 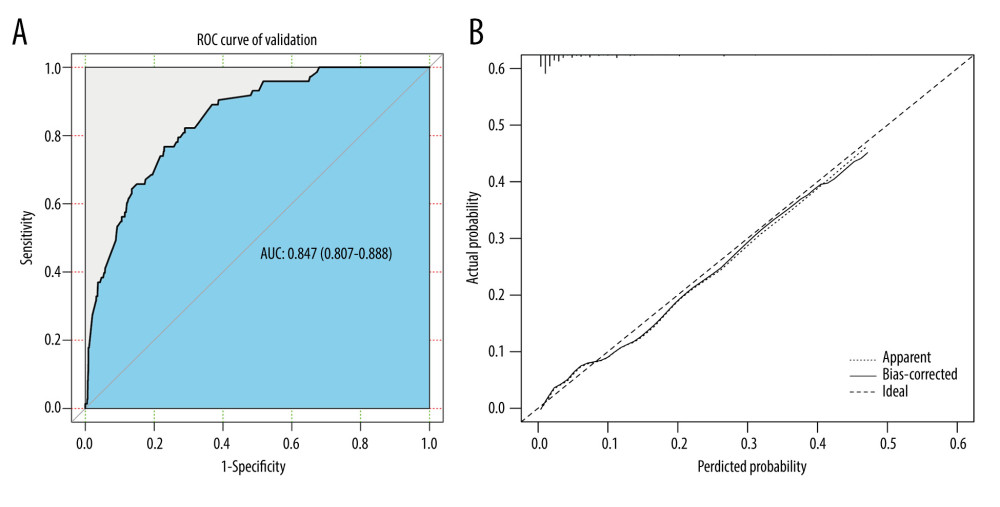 Figure 6. The (A) ROC curves and (B) calibration curve of the external validation set. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation.
Figure 6. The (A) ROC curves and (B) calibration curve of the external validation set. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation. Tables
Table 1. Baseline characteristics of study populations [n (%)/M (Q1, Q3)].![Baseline characteristics of study populations [n (%)/M (Q1, Q3)].](https://jours.isi-science.com/imageXml.php?i=t1-medscimonit-28-e933379.jpg&idArt=933379&w=1000) Table 2. Results of univariate analysis in the training set.
Table 2. Results of univariate analysis in the training set.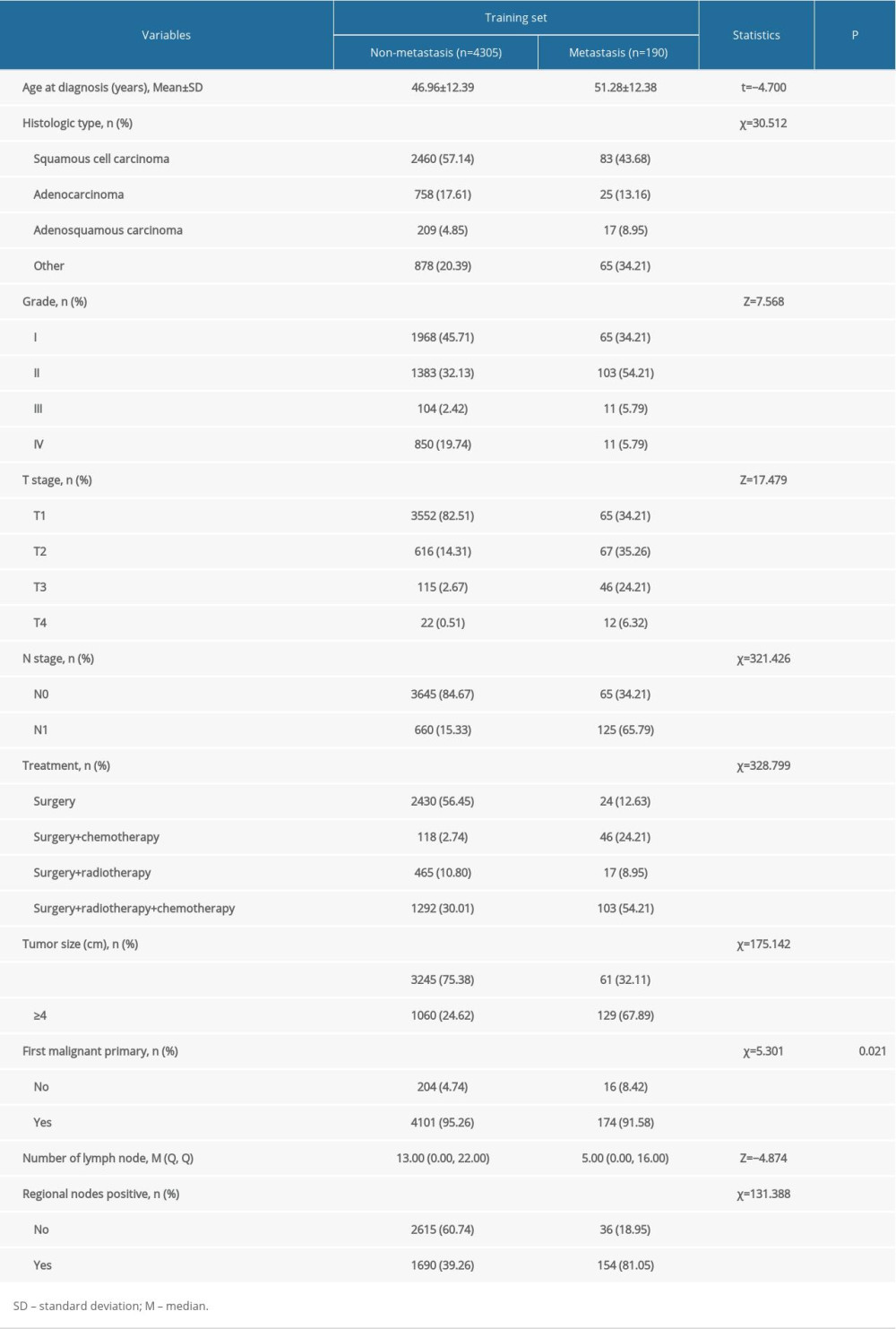 Table 3. Results of the McNemar’s test in the internal validation set.
Table 3. Results of the McNemar’s test in the internal validation set.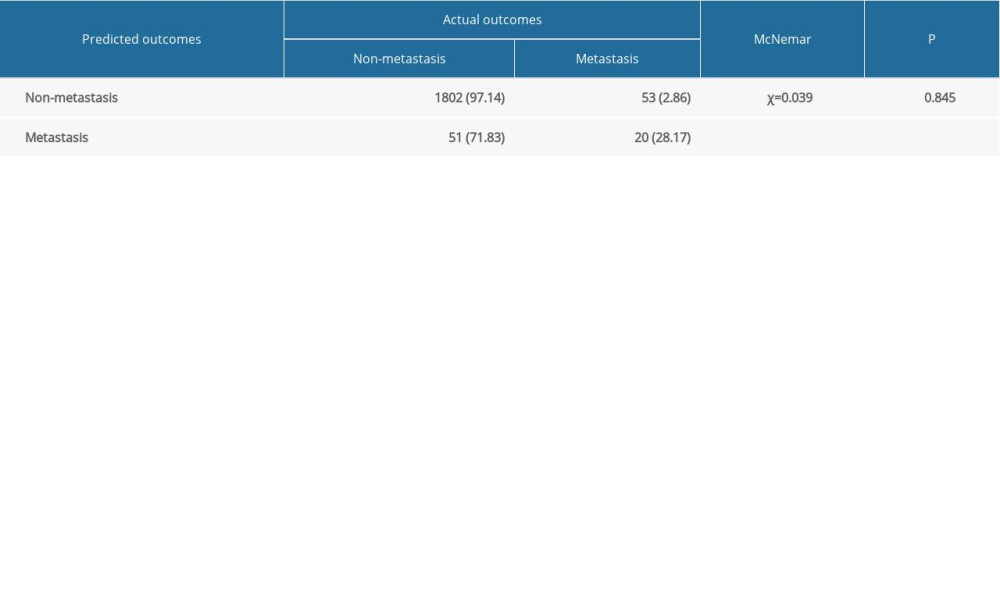 Table 4. Results of the McNemar’s test in the external validation set.
Table 4. Results of the McNemar’s test in the external validation set.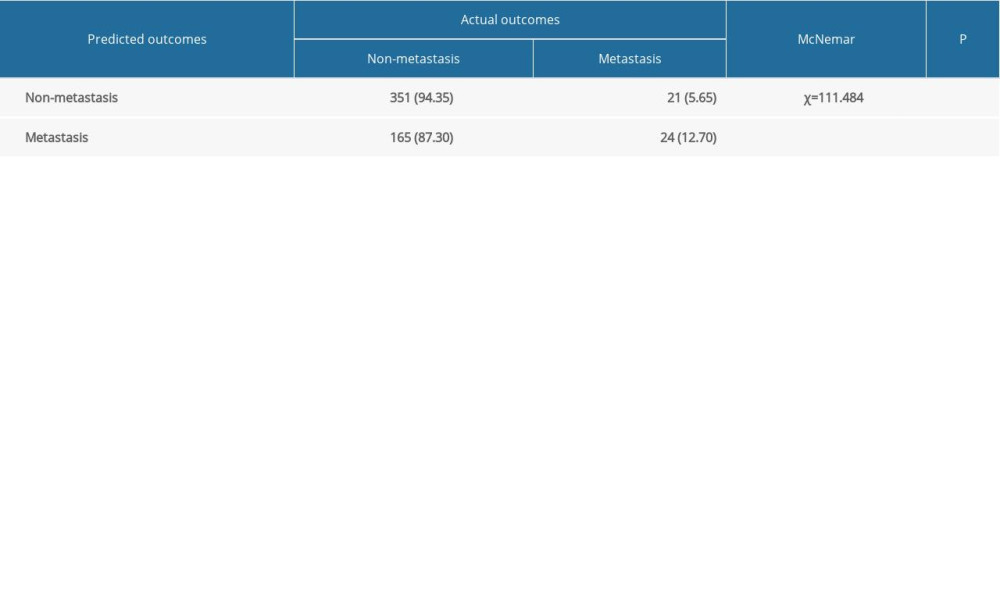 Table 5. Results of subgroup analysis in the external validation set.
Table 5. Results of subgroup analysis in the external validation set.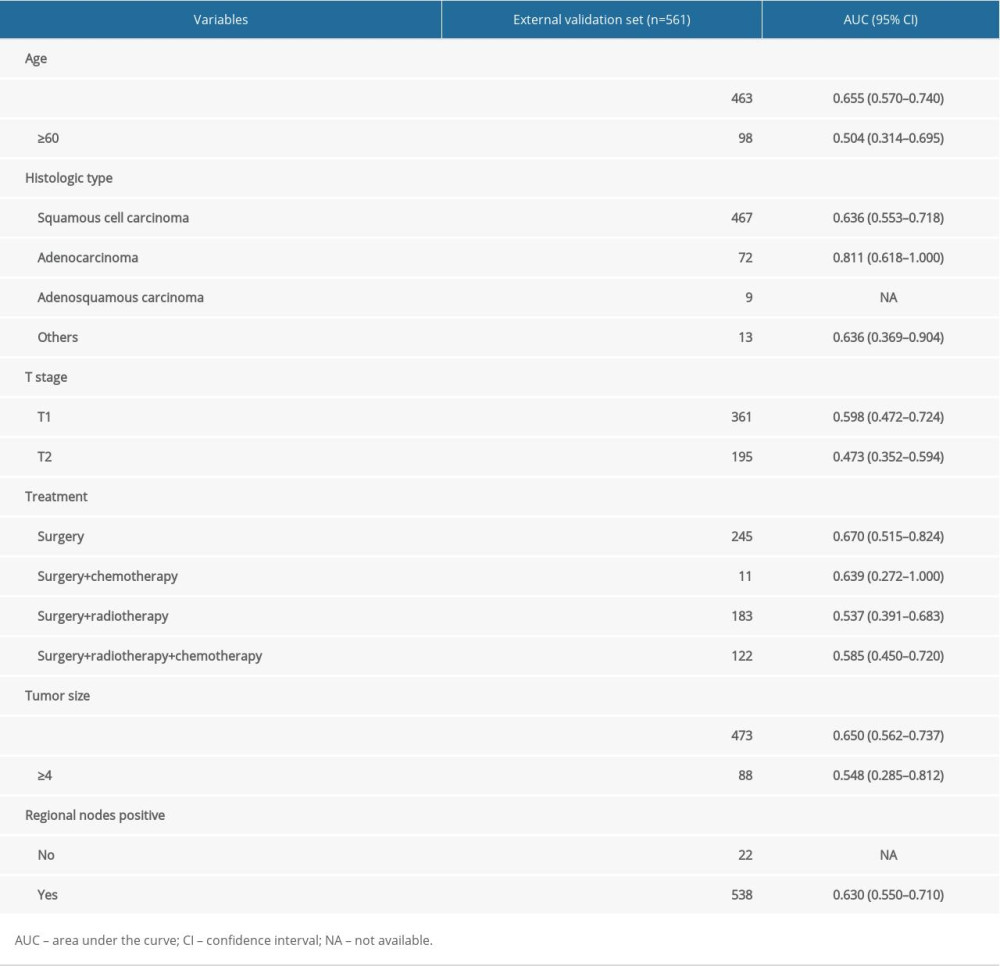
References
1. Castle PE, Einstein MH, Sahasrabuddhe VV, Cervical cancer prevention and control in women living with human immunodeficiency virus: Cancer J Clin, 2021; 71; 505-26
2. Bray F, Ferlay J, Soerjomataram I, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2018; 68; 394-424
3. Lyu Y, Ding L, Gao T, Influencing factors of high-risk human papillomavirus infection and dna load according to the severity of cervical lesions in female coal mine workers of China: J Cancer, 2019; 10; 5764
4. Burki TK, Outcomes after minimally invasive surgery in cervical cancer: Lancet Oncol, 2018; 19; e674
5. Huang B-x, Fang F, Progress in the study of lymph node metastasis in early-stage cervical cancer: Curr Med Sci, 2018; 38; 567-74
6. Zhang X, Sheng X, Yan Y, Clinical pathological factors of radiosensitivity in patients with cervical cancer treated with radiotherapy: Chin J Cancer Prev Treat, 2011; 18; 1204-7
7. Alfsen GC, Kristensen GB, Skovlund E, Histologic subtype has minor importance for overall survival in patients with adenocarcinoma of the uterine cervix: A population based study of prognostic factors in 505 patients with nonsquamous cell carcinomas of the cervix: Cancer, 2001; 92; 2471-83
8. Liu MT, Hsu JC, Liu WS, Prognostic factors affecting the outcome of early cervical cancer treated with radical hysterectomy and postoperative adjuvant therapy: Eur J Cancer Care (Engl), 2008; 17; 174-81
9. Kim HJ, Rhee WJ, Choi SH, Clinical outcomes of adjuvant radiation therapy and prognostic factors in early stage uterine cervical cancer: Radiat Oncol J, 2015; 33; 126-33
10. Kim MK, Jo H, Kong HJ, Postoperative nomogram predicting risk of recurrence after radical hysterectomy for early-stage cervical cancer: Int J Gynecol Cancer, 2010; 20; 1581-86
11. Gülseren V, Kocaer M, Çakır İ, Postoperative nomogram for the prediction of disease-free survival in lymph node-negative stage I–IIA cervical cancer patients treated with radical hysterectomy: J Obstet Gynaecol, 2020; 40; 699-704
12. Je HU, Han S, Kim YS, Risk prediction model for disease-free survival in women with early-stage cervical cancers following postoperative (chemo)radiotherapy: Tumori, 2018; 104; 105-10
13. Tang X, Guo C, Liu S, A novel prognostic nomogram utilizing the 2018 FIGO staging system for cervical cancer: A large multicenter study: Int J Gynaecol Obstet, 2021; 155; 86-94
14. Lee Y-J, Kim D-Y, Lee S-W, A postoperative scoring system for distant recurrence in node-positive cervical cancer patients after radical hysterectomy and pelvic lymph node dissection with para-aortic lymph node sampling or dissection: Gynecol Oncol, 2017; 144; 536-40
15. Cao L, Sun PL, He Y, Immune stromal features in cervical squamous cell carcinoma are prognostic factors for distant metastasis: A retrospective study: Pathol Res Pract, 2020; 216; 152751
16. Schmid MP, Franckena M, Kirchheiner K, Distant metastasis in patients with cervical cancer after primary radiotherapy with or without chemotherapy and image guided adaptive brachytherapy: Gynecol Oncol, 2014; 133(2); 256-62
17. Cao L, Sun PL, He Y, Desmoplastic reaction and tumor budding in cervical squamous cell carcinoma are prognostic factors for distant metastasis: A retrospective study: Cancer Manag Res, 2020; 12; 137-44
18. Je HU, Han S, Kim YS, A nomogram predicting the risks of distant metastasis following postoperative radiotherapy for uterine cervical carcinoma: A Korean radiation oncology group study (KROG 12-08): Radiother Oncol, 2014; 111; 437-41
19. Yoon WS, Yang DS, Lee JA, Validation of nomograms for survival and metastases after hysterectomy and adjuvant therapy in uterine cervical cancer with risk factors: Biomed Res Int, 2017; 2017; 2917925
20. Dong D, Tang L, Li ZY, Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer: Ann Oncol, 2019; 30; 431-38
21. Wu S, Zheng J, Li Y, A radiomics nomogram for the preoperative prediction of lymph node metastasis in bladder cancer: Clin Cancer Res, 2017; 23; 6904-11
22. Guo CG, Zhao DB, Liu Q, A nomogram to predict lymph node metastasis in patients with early gastric cancer: Oncotarget, 2017; 8; 12203
23. D’Alterio C, Scala S, Sozzi G, Paradoxical effects of chemotherapy on tumor relapse and metastasis promotion: Semin Cancer Biol, 2020; 60; 351-61
24. Guruvayoorappan C, Tumor versus tumor-associated macrophages: How hot is the link?: Integr Cancer Ther, 2008; 7; 90-95
25. Burke B, Giannoudis A, Corke KP, Hypoxia-induced gene expression in human macrophages: Implications for ischemic tissues and hypoxia-regulated gene therapy: Am J Pathol, 2003; 163; 1233-43
26. Gocheva V, Wang H-W, Gadea BB, IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion: Genes Dev, 2010; 24; 241-55
27. Wyckoff J, Wang W, Lin EY, A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors: Cancer Res, 2004; 64; 7022-29
28. Goswami S, Sahai E, Wyckoff JB, Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop: Cancer Res, 2005; 65; 5278-83
29. Du R, Lu KV, Petritsch C, HIF1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion: Cancer Cell, 2008; 13; 206-20
30. Su S, Liu Q, Chen J, A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis: Cancer Cell, 2014; 25; 605-20
31. Dijkgraaf EM, Heusinkveld M, Tummers B, chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment: Cancer Res, 2013; 73; 2480-92
32. Sundahl N, Duprez F, Ost P, Effects of radiation on the metastatic process: Mol Med, 2018; 24; 1-20
33. Madani I, De Neve W, Mareel M, Does ionizing radiation stimulate cancer invasion and metastasis?: Bull Cancer, 2008; 95; 292-300
34. Lee SY, Jeong EK, Ju MK, Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation: Mol Cancer, 2017; 16; 10
35. Rüegg C, Monnier Y, Kuonen F, Imaizumi N, Radiation-induced modifications of the tumor microenvironment promote metastasis: Bull Cancer, 2011; 98; E47-57
36. Sofia Vala I, Martins LR, Imaizumi N, Low doses of ionizing radiation promote tumor growth and metastasis by enhancing angiogenesis: PLoS One, 2010; 5; e11222
37. Turan T, Yildirim BA, Tulunay G, Prognostic effect of different cut-off values (20 mm, 30 mm and 40 mm) for clinical tumor size in FIGO stage IB cervical cancer: Surg Oncol, 2010; 19; 106-13
38. Viswanathan AN, Erickson B, Gaffney DK, Comparison and consensus guidelines for delineation of clinical target volume for CT-and MR-based brachytherapy in locally advanced cervical cancer: Int J Radiat Oncol Biol Phys, 2014; 90; 320-28
39. Attaleb M, Khyatti M, Benbacer L, Status of p16INK4a and E-cadherin gene promoter methylation in moroccan patients with cervical carcinoma: Oncol Res, 2009; 18; 185-92
40. Wertheim MS, Hakes T, Daghestani A, A pilot study of adjuvant therapy in patients with cervical cancer at high risk of recurrence after radical hysterectomy and pelvic lymphadenectomy: J Clin Oncol, 1985; 3; 912-16
41. Uno T, Ito H, Itami J, Postoperative radiation therapy for stage IB–IIB carcinoma of the cervix with poor prognostic factors: Anticancer Res, 2000; 20; 2235-39
42. Wang C, Yang C, Wang W, A prognostic nomogram for cervical cancer after surgery from SEER database: J Cancer, 2018; 9; 3923-28
43. Liu X, Meng Q, Wang W, Predictors of distant metastasis in patients with cervical cancer treated with definitive radiotherapy: J Cancer, 2019; 10; 3967
Figures
 Figure 1. Flow chart for screening included patients with cervical cancer. Draw.io (version 12.6.5.330, JGraph Ltd.) was used for figure creation.
Figure 1. Flow chart for screening included patients with cervical cancer. Draw.io (version 12.6.5.330, JGraph Ltd.) was used for figure creation. Figure 2. Results of multivariate analysis. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation.
Figure 2. Results of multivariate analysis. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation. Figure 3. Nomogram prediction of postoperative metastasis. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation.
Figure 3. Nomogram prediction of postoperative metastasis. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation. Figure 4. ROC curve of the predictive nomogram. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation.
Figure 4. ROC curve of the predictive nomogram. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation. Figure 5. The (A) ROC curves and (B) calibration curve of the internal validation set. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation.
Figure 5. The (A) ROC curves and (B) calibration curve of the internal validation set. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation. Figure 6. The (A) ROC curves and (B) calibration curve of the external validation set. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation.
Figure 6. The (A) ROC curves and (B) calibration curve of the external validation set. R software (version 4.0.2, R Foundation for Statistical Computing) was used for figure creation. Tables
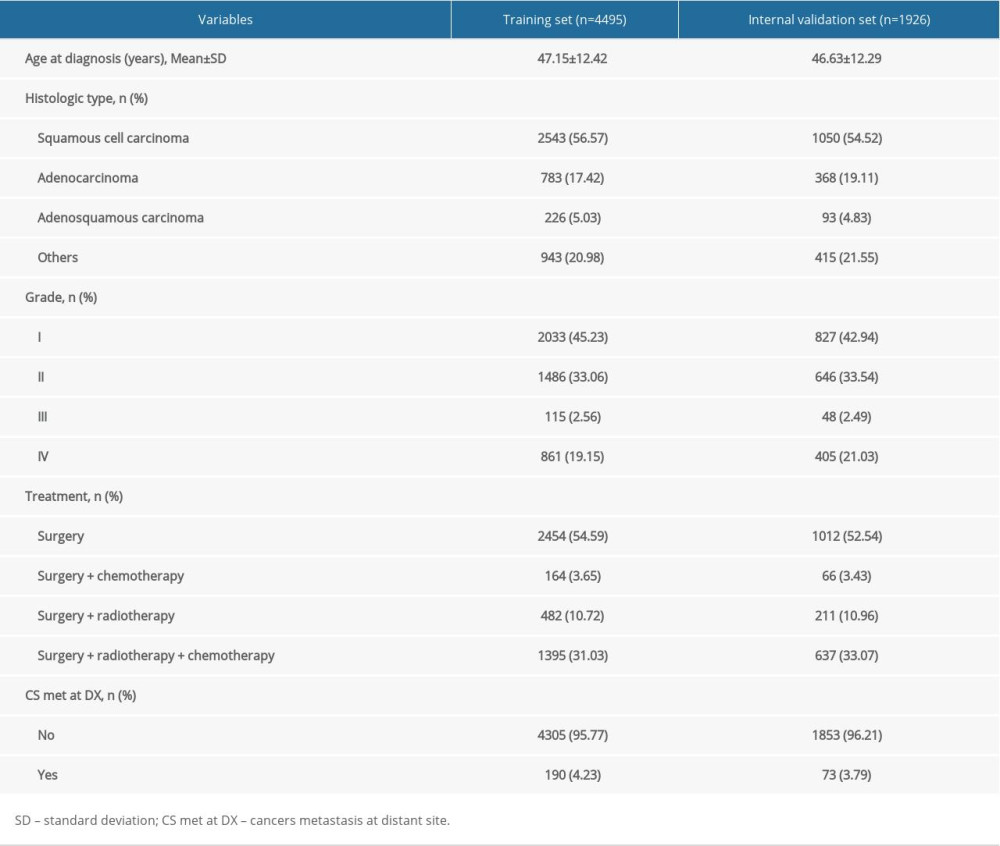 Table 1. Baseline characteristics of study populations [n (%)/M (Q1, Q3)].
Table 1. Baseline characteristics of study populations [n (%)/M (Q1, Q3)]. Table 2. Results of univariate analysis in the training set.
Table 2. Results of univariate analysis in the training set. Table 3. Results of the McNemar’s test in the internal validation set.
Table 3. Results of the McNemar’s test in the internal validation set. Table 4. Results of the McNemar’s test in the external validation set.
Table 4. Results of the McNemar’s test in the external validation set. Table 5. Results of subgroup analysis in the external validation set.
Table 5. Results of subgroup analysis in the external validation set. Table 1. Baseline characteristics of study populations [n (%)/M (Q1, Q3)].
Table 1. Baseline characteristics of study populations [n (%)/M (Q1, Q3)]. Table 2. Results of univariate analysis in the training set.
Table 2. Results of univariate analysis in the training set. Table 3. Results of the McNemar’s test in the internal validation set.
Table 3. Results of the McNemar’s test in the internal validation set. Table 4. Results of the McNemar’s test in the external validation set.
Table 4. Results of the McNemar’s test in the external validation set. Table 5. Results of subgroup analysis in the external validation set.
Table 5. Results of subgroup analysis in the external validation set. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








