12 February 2022: Clinical Research
Autonomic Tone Variations Prior to Onset of Paroxysmal Atrial Fibrillation
Xia Zhong1ABCDEF*, Huachen Jiao2ACDFG, Jinchao Gao3ABCD, Jing Teng1DEFDOI: 10.12659/MSM.934028
Med Sci Monit 2022; 28:e934028
Abstract
BACKGROUND: Variations of heart rate variability (HRV) before paroxysmal atrial fibrillation (PAF) onset are still controversial. We aimed to observe the autonomic tone variations before PAF onset based on HRV analysis.
MATERIAL AND METHODS: We prospectively investigated 24-h Holter recordings of 60 patients with PAF (M/F: 34/26) and 40 healthy people in sinus rhythm (M/F: 18/12). According to clinical information and Poincaré scatter plot, 60 PAF patients were divided into sympathetic group (n=20) and vagus group (n=40). Time domain and frequency domain parameters of HRV were respectively measured before PAF episodes in 3 subgroups. Five time periods were studied using the ANOVA.
RESULTS: No significant variations were observed for the HRV parameters during 60 minutes preceding PAF in sympathetic group. A significant and linear change in SDNN, RMSSD, PNN50, HF and LF/HF during 60 minutes preceding PAF onset in vagus group. Compared with controls, RMSSD, LF and HF were significantly longer in patients with PAF during 60 minutes before PAF. Comparing sympathetic group and vagus group, we observed the same pattern of autonomic variations with a progressive decrease in LF and HF. A progressive decrease in PNN50 and LF/HF of sympathetic group and a significant increase in PNN50 and LF/HF of vagus group were also observed.
CONCLUSIONS: Patients with PAF mediated by different autonomic nerves have HRV variations, especially vagus PAF, there was a progressive increase with vagal tone during 60 minutes before PAF onset. The findings may help clinicians better intervene in PAF.
Keywords: Arrhythmias, Cardiac, Atrial Fibrillation, Heart Rate Determination, Aged, 80 and over, Circadian Rhythm, Echocardiography, Electrocardiography, Ambulatory, Female, Follow-Up Studies, Heart Rate, Humans, Male, Prospective Studies, Time Factors, Vagus Nerve
Background
Atrial fibrillation (AF) is an increasingly prevalent arrhythmia with significant health and socioeconomic impact. Currently, AF is affecting 33 million people worldwide [1]. Although ablation approach and antiarrhythmic drugs as effective therapies for AF are widely used, recurrence rates and mortality rates still remain high. In addition, evidence suggests that the current long-term outcome of catheter ablation of AF is not ideal, especially for patients with paroxysmal atrial fibrillation (PAF), the single-procedure success rate is only 66.6% [2], and the 5-year success rate is less than 30% after a single procedure [3]. Given the increasing risk of morbidity and mortality associated with AF, as well as its significant health and socioeconomic impacts, the prevention and treatment of AF cannot be ignored.
AF is an arrhythmia with complicated mechanisms, and its pathophysiology is unclear [4], which have impeded advances in treatment of AF. Although some progress has been made in animal experiments and clinical research, the specific pathophysiologic mechanism of AF is still poorly understood. It is well known that the heart is abundantly innervated by the cardiac nerves in humans [5], especially near PV ostia [6], that is, the LA within 5 mm of the PV-LA junction [7]. There, cholinergic and adrenergic nerves are strongly associated in the epicardium, suggesting that activation and remodeling of CANS is associated with the initiation and maintenance of AF [8]. In this regard, a few previous studies sought to demonstrate this mechanism. In 1978, Coumel investigated the role of the central autonomic nervous system (CANS) in the onset of AF based on Holter recordings, which helps distinguish the 2 types of AF onset, namely vagal and adrenergic [9,10]. In recent decades, autonomic changes before the onset of PAF have been observed; an increasing trend in sympathetic tone or a weakening trend vagal tone has been observed before AF, and there is increasing evidence that sympathetic nerves and the vagus nerve can initiate AF separately or in concert in regulating the electrophysiological properties of the atrium [8]. Furthermore, evidence also shows that most isolated cases of paroxysmal AF are vagus-dependent, whereas sympathetic-dependent AF is often accompanied by organic cardiovascular diseases [8]. Accordingly, the imbalance between the sympathetic nerve and the vagus nerve is considered to be the major mechanism for AF onset, and the CANS appears to be the target for modulation of AF [8]. Nowadays, CANS is also increasingly recognized as an important trigger mechanism for AF.
Heart rate variability (HRV) is recognized as a useful tool to evaluate sympathetic and parasympathetic influences on disease states [9]. HRV analyzes variation in beat intervals or, correspondingly, in the instantaneous heart rate (HR) [10], which is the degree of sinus arrhythmia. As a non-stationary signal, the variation of HR can reflect many heart diseases. The normal variation of HR depends on the neural regulation of the cardiac and circulatory system autonomic nerve [11], as well as the balance of the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS) influences on HR. HRV is measured by investigating the variations of instantaneous HR and is generally assessed by time and spectrum analytical methods [12]. Currently, the distinction of sympathetic nerve- and vagus nerve-dependent AF is mainly based on HRV analysis of ECG recordings. However, the evidence provided by these parameters for human autonomic nerve activity recording is still inadequate. Although previous studies also attempted to investigate the different variations of HRV prior to the onset of AF, conflicting conclusions were reported [13–15]. Moreover, due to the undefined reference value, the exploration of HRV can still only be compared within a group or individual in longitudinal studies [16]. Therefore, further studies are needed to explore the effects of CANS sympathetic nerve and vagal nerve in AF initiation.
AF is a progressive disease that occurs as 3 major patterns in human: paroxysmal atrial fibrillation, persistent atrial fibrillation, and permanent atrial fibrillation [8]. Paroxysmal atrial fibrillation (PAF) is the beginning of AF in a self-terminating paroxysmal form [17]. With time, it is likely that PAF can evolve to become the persistent pattern. In this regard, we feel that studying autonomic changes preceding PAF onset will make important contributions to the prevention and treatment of AF. In the present study, we sought to analyze HRV and to assess alterations in autonomic tone preceding PAF episodes, which may contribute to the better understanding of PAF pathogenesis.
Material and Methods
STUDY POPULATION:
This was a prospective observational study conducted at the Affiliated Hospital of Shandong University of Traditional Chinese Medicine from January 2018 to December 2020. We enrolled 60 PAF patients (M/F: 34/26, ages 49–85 years) and 40 healthy people in sinus rhythm (M/F: 18/12, ages 47–82 years). According to guidelines [18], AF attacks are considered to be an arrhythmia lasting long enough for a 12-lead ECG to be recorded, or lasting for at least 30 s. In addition, PAF was specifically defined as AF that terminates spontaneously or with intervention within 7 days of initiation. In this study, device-detected episodes of PAF were evaluated by 2 experienced cardiologists. Based on medical history, clinical examination, and 12-lead resting ECG, the presence and type of structural heart disease were assessed. Each case must have at least 1 episode of sustained PAF (>30 s). We excluded patients with persistent or permanent AF and malignant arrhythmias such as ventricular tachycardia and ventricular fibrillation. patients with sinus node dysfunction or abnormal atrioventricular conduction were not included. Patients taking psychotropic drugs within 48 h were not included. In addition, patients younger than 18 years and older than 85 years and pregnant women were also excluded. Before participating in this study, all subjects provided signed informed consent. The research protocol complied with the Declaration of Helsinki and was approved by the Ethics Committee of Shandong University of Traditional Chinese Medicine in China.
ECHOCARDIOGRAPHY EXAMINATION:
Conventional transthoracic echocardiography was performed to determine the left atrial end systolic anteroposterior diameter (LAD) using the GE Vivid7 Dimension system (General Electric Company, Fairfield, CT, USA) by experienced physicians in the Ultrasound Department of our hospital. Based on medical history, clinical examination, and 12-lead resting ECG, the presence and type of structural heart disease were assessed.
HRV ANALYSES:
All subjects underwent 24-h Holter monitoring. During monitoring, patients were required to perform their normal daily activities. Continuous ECG recordings of 60 subjects were obtained by 2 experienced doctors using a 3-channel 24-h Holter system (MIC3H-3L, Jinco Medical Equipment Company, Beijing, China). The acquisition sampling rate was 4000 Hz. Recordings were extracted from the 24-h Holter data and cleaned. According to standardized guidelines, HRV analysis was performed [19]. HRV features of 24-h Holter monitoring at different timepoints before PAF onset were investigated. Five timepoints were selected for HRV analyses: 60 min, 45 min, 30 min, 15 min before the onset of PAF, and at onset. Importantly, selected Holter recordings had to meet the following criteria: (1) Normal sinus rhythm is at least 50% during recording period; (2) One or more episodes of sustained AF (≥30 s); (3) Sinus rhythm sustained for at least 1 h before the onset of AF; (4) No or few artifacts 1 h prior to the onset of AF; (5) 75% of N-N intervals appear during the period of recording; and (6) Continuous recording for more than 18 h. In addition, all episodes of PAF were manually identified and labeled at the speed of 25 mm/s. All subjects received the 60-min HRV analysis in the same period as the PAF onsets.
HRV analyses were performed capturing standard parameters from time and frequency domains in daytime and nighttime. Data were obtained by 2 experienced doctors in a double-blind fashion at a rate of 128 samples per second. Then, the heartbeat (RR) interval was calculated and exported to an IBM PC-compatible computer. All parameters of HRV were based on 24-h Holter monitoring as well as from windowed analyses. Specifically, from successive 5-min windows, we calculated and analyzed the time and frequency domain parameters and then averaged them over a 24-h period. In fact, time domain parameters were derived from the RR intervals [20]. We tested each QRS interval and determined the normal-to-normal (RR) interval. Four time domain indexes were selected: SDNN, RMSSD, NN50, and PNN50. Further, 3 frequency domain indexes were derived: LF, HF, and LF/HF. All parameters were measured in normalized units. A list of parameters with details is provided in Table 1.
STATISTICAL ANALYSIS:
Statistical analyses were performed with SPSS software (Version 26.0, Chicago, IL, USA). All measures are expressed as mean±SD and n(%). The
Results
BASELINE CHARACTERISTICS OF POPULATION:
The baseline characteristics of the study population are shown in Table 2. Sixty PAF patients (M/F: 34/26, ages 49–85 years, sinus rate: 81±17 bpm) and 40 controls (M/F: 24/16, ages 48–82 years, sinus rate: 79±15 bpm) were included. Compared with controls, patients with PAF had significantly larger diameter of the left atrium (40.53±2.20 vs 39.50±2.49 mm, P=0.03). There were no statistically significant differences between the 2 groups in other baseline characteristics (age, sex, smokers, drinkers, structural heart disease, family history, initial diagnosis, P>0.05).
SUBGROUP CLASSIFICATION OF THE STUDY POPULATION:
According to the Poincaré scatter plots automatically generated in Holter monitoring (Figure 1), among the 40 subjects in the control group, there were 36 cases of comet shape, 3 cases of irregular shape, and 1 case of oval shape. We conducted a basic assessment of PAF patients based on electrocardiogram, recognized triggers, associated features, and combined with the shape of the scatter plot to distinguish different types of autonomic PAF (Figure 2). The 60 PAF patients were divided into 2 subgroups: the sympathetic group was dominated by patients with spider-shaped (n=20, M/F: 14/6, mean age 68.25±9.48 years) and the vagus group was dominated by patients with oval, spider+oval, and irregular shape (n=40, M/F: 20/20, mean age 73.27±9.23 years). There was no statistically significant difference between the 2 groups in baseline characteristics (age, sex, smokers, drinkers, structural heart disease, family history, and initial diagnosis).
VARIATIONS IN HRV PARAMETERS BEFORE PAF ONSET IN SYMPATHETIC GROUP:
As shown in Table 3 and Figure 3, no significant variations were observed for the HRV parameters during the 60 min preceding PAF, but there was a trend toward a progressive increase in the SDNN (from 54.40±43.41 to 70.80±33.19 ms; P=0.57; Figure 3A), RMSSD (from 65.10±54.67 to 86.15±45.51 ms; P=0.61; Figure 3B), and LF (from 261.68±258.34 to 573.53±493.01 ms; P=0.06; Figure 3C) until 60 min preceding PAF. In addition, we observed a sharp decrease in HF during the 30 min before PAF (from 341.56±491.51 to 293.27±295.23 ms2; P=0.75; Figure 3D).
VARIATIONS IN HRV PARAMETERS BEFORE PAF ONSET IN VAGUS GROUP:
As shown in Table 4 and Figure 4, there was a significant and linear change in the SDNN, RMSSD, PNN50, HF, and LF/HF during the 60 min preceding PAF onset. Specifically, there was a progressive increase in SDNN during the 60 min before PAF onset (from 65.98±54.19 to 142.73±54.37 ms; P<0.001; Figure 4A). There was a sharp decrease in RMSSD during the 60 to 45 min before PAF onset (from 100.23±90.76 to 90.40±76.10 ms; P<0.001; Figure 4B) and a progressive increase in RMSSD during the 45 min before PAF onset (from 90.40±76.10 to 170.43±94.40 ms; P<0.001; Figure 4B). There was a slight decrease in PNN50 during the 60 to 45 min before PAF onset (from 7.89±9.33 to 7.84±9.92%; P<0.001; Figure 4C) and a progressive increase in the PNN50 during the 45 min before PAF onset (from 7.84±9.92 to 16.29±9.30%; P<0.001; Figure 4C). Moreover, there was a sharp decrease in HF during the 60 to 45 min before PAF onset (from 533.55±789.35 to 463.92±650.44 ms2; P=0.66; Figure 4D) and a progressive increase during the 45 min before PAF onset (from 463.92±650.44 to 1280.93±982.64 ms2; P<0.001; Figure 4D), and there was a progressive decrease in LF/HF during the 60 min before PAF onset (from 1.56±1.44 to 0.55±0.19; P=0.01).
COMPARISON OF HRV PARAMETERS BETWEEN PATIENTS WITH PAF AND CONTROLS:
As shown in Table 5 and Figures 5–9, compared with controls, SDNN was significantly longer in patients with PAF during the 15 min before PAF (P=0.04) and at onset (P<0.001), the PNN50 was significantly longer in patients with PAF at onset (P<0.001), and RMSSD, LF, and HF were significantly longer in patients with PAF during the 60 min before PAF (P<0.05), especially in the vagus group. In addition, LF/HF was significantly shorter in patients with vagally-mediated PAF and was significantly longer in patients with sympathetic-mediated PAF during the 15 min before PAF (P=0.03) and at onset (P<0.001).
Discussion
ANS is one of 3 components of Coumel’s triangle, which is of major importance for the onset of AF [14]. In recent years, sufficient evidence on the mechanism of AF occurrence has been accumulated. Nevertheless, CANS is still an important topic in the research field of AF pathophysiology. It is well known that the sympathetic nerve and the vagus nerve are 2 important branches influencing autonomic nerve activity. Their action can be expressed as simultaneous excitement or reduced activity, or one of them is weakened or strengthened by the other or strengthened by each other [21]. Previous studies have confirmed that sympathetic nerves that induce and maintain AF tend to perform automaticity and delay afterpotentials [22], whereas vagus nerves favor shortening the atrial refractory period and promoting reentry [23,24]. Another study also demonstrated that sympathetic nerves promote AF by increasing calcium transients in the synapses [25], and activated adrenergic signal pathways increased neurotransmitter release. Accordingly, it is indisputable that ANS plays a complex and important role in AF onset, particularly variations of sympathetic and vagal balance.
There is growing evidence that HRV is a non-invasive technique and a useful indicator for monitoring variations of ANS. Although a few clinical studies have analyzed changes in autonomic tone prior to AF onset, the measurements are still controversial. A previous study reported that HRV is related to vagal tone in patients with AF [26]. Chou et al [27] indicated that although AF is triggered by sympathetic nerves, the vagus nerve plays dominant role in initiation of PAF. Bettoni et al [28] found a significant increase in adrenergic predominance at the 20 min preceding PAF onset, together with a shift toward more vagal predominance prior to PAF attack in patients with and without structural heart disease. Fioranelli et al [29] found a trend toward a significant increase in adrenergic tone during the 5 min before PAF onset. Indeed, in most instances, vagal influences are predominant, and sympathetically-mediated AF is less common during the onset of PAF [30].
In the present study, we observed both sympathetic (33.33%) and vagal (66.67%) forms of PAF onset, without any correlation with age, sex, smoking, drinking, structural heart disease, family history, or initial diagnosis. In contrast to previous studies, we used the Poincaré scatter plot to assist in classifying PAF. The Poincaré scatter plot is a non-digital and non-linear method used in HRV analysis. Specifically, the Poincaré plot, which a visual graph, can describe the non-linear relationship of RRI by plotting 2 adjacent RRIs and measures heart rate (HR) neuromodulation non-linearly [31]. Through the assessing the size and shape of the scatter plot, we can evaluate the size of HRV and the type of arrhythmia, and the abundant data and clear plot provide higher credibility. In the present study, we used a computer to automatically measure the RR interval, using the previous RR interval of 2 adjacent heartbeats as the X axis, and the RR interval of the next heartbeat as the Y axis to draw a point, and continue to draw in this way to form a Poincaré scatter plot. Generally speaking, different heart rhythms show different shapes of scatter plots. According to our study results, the Poincaré scatter plot of healthy people in sinus rhythm is mainly in the shape of a comet. The sympathetic group is dominated by patients with a spider-shaped plot, the vagus group is dominated by patients with oval-, spider+oval-, and irregularly-shaped plots. Some previous studies [31–33] support this observation.
Although multiple studies [4,28] have reported on ANS activity before PAF attacks, there seem to be no studies using Poincaré plots to compare the HRV changes of sympathetic PAF and vagus PAF. In our study, HRV analysis was performed during the 60 min prior to PAF onset. We mainly drew the conclusions from the time threshold and frequency domain parameters. SDNN, RMSSD, PNN50 mainly represent the time domain, and LF, HF, and LF/HF mainly represent the frequency domain in HRV analysis. Time domain parameters of HRV represent the amount of variability in measurements of the inter-beat interval (IBI) [34], SDNN represents a comprehensive interaction between sympathetic tone and vagus tone, and RMSSD represents the beat-to-beat changes in HR and reflects vagally-mediated changes in HRV [35]. Indeed, the evidence suggests that the performance of frequency domain parameters is clearer and superior [19]. It is widely believed that vagal activity is beneficial to HF. Conversely, the activity of sympathetic nerves is beneficial to LF, especially as related to breathing [36]. HF reflects parasympathetic activity and is also related to respiration [37]. In addition, HF power shows a pattern of increasing at night and decreasing during the day [38]. LF/HF is generally considered as a signal of balancing sympathetic nerve and vagus nerve tone.
Our HRV analysis showed significant variations in sympathetic and vagal balance in the last 60 min before PAF episodes. Our results indicate that in the sympathetic group, no significant variations were observed for the HRV parameters during the 60 min preceding PAF. We speculated that this is related to the short-term HRV recordings, small size of the group, and data characteristics. Nevertheless, we observed a trend toward a progressive increase in LF/HF during the 60 to 45 min preceding PAF, followed by a progressive decrease during the 45 to 30 min preceding PAF, together with a progressive increase during the 30 min before PAF, suggesting a primary increase in vagal tone followed by an increase in sympathetic tone, and then another increase in vagal predominance. In the vagus group, although we found no significant variations in LF during the 60 min before PAF onset, there was a trend toward a progressive decrease during the 60 to 30 min before PAF onset and a progressive increase during the 30 min before PAF onset, suggesting an increase and then a decrease in sympathetic tone. Moreover, a significant decrease in HF during the 60 to 45 min before PAF onset and a significant increase during the 45 min before PAF onset were observed, suggesting a decrease followed by an increase in vagal tone. In addition, a progressive decrease in LF/HF during the 60 min before PAF onset was observed, suggesting an increase in vagal tone.
Our study has some limitations that should be considered. First, we could not clearly identify the clinical history of patients and factors that may contribute to PAF, and patients with diseases that may involve damage to the autonomic nervous system were also not identified. However, we tried our best to maintain small differences in the clinical data of the control group. Although the apparent heterogeneity of patients enrolled in relation to the underlying structural heart disease and complications is the major limitation of this study, Bettoni et al [28] reported that there was no significant difference in HRV changes between patients with “lone” PAF and structural heart disease. Second, due to strict criteria of inclusion and exclusion, the study included a small number of subjects. Third, during Holter monitoring, due to ethical reasons, it was impossible to withdraw all antiarrhythmic drugs, which may have biased the results of HRV analysis. In addition, it should also be considered that Holter recordings were performed without documentation of physical activity or synchronization of feeding time or time when the subject went to bed. Finally, the factor of patient compliance cannot be ignored.
Conclusions
In conclusion, we investigated autonomic tone different variations before PAF onset based on HRV analysis. We observed that patients with PAF mediated by different autonomic nerves have HRV variations, and vagus PAF in particular, and there was a trend toward a progressive increase with vagal tone and a progressive decrease with sympathetic tone during the 60 min before PAF onset. A better understanding of the changes in autonomic tone before the onset of PAF mediated by different autonomic nerves can contribute to developing specific intervention strategies.
Figures
 Figure 1. Poincaré dispersed-dot plot of PAF. (A) Oval shape, (B) spider shape, (C) irregular shape, (D) comet shape.
Figure 1. Poincaré dispersed-dot plot of PAF. (A) Oval shape, (B) spider shape, (C) irregular shape, (D) comet shape. 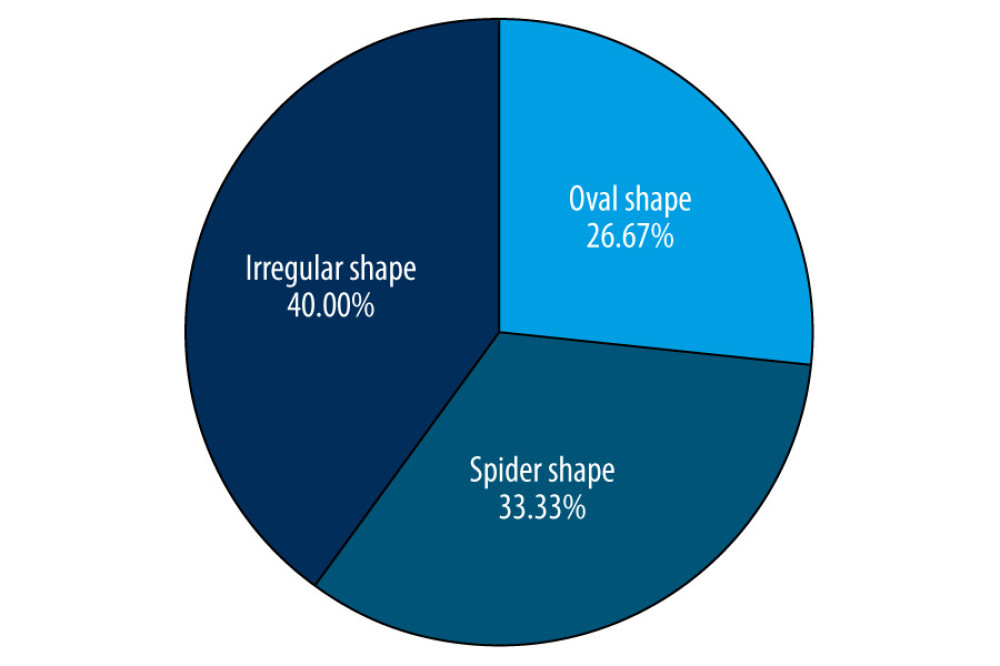 Figure 2. Distribution of different shapes of Poincaré dispersed-dot plot for PAF group. The figure was created using GraphPad Prism software (version 9.0.0).
Figure 2. Distribution of different shapes of Poincaré dispersed-dot plot for PAF group. The figure was created using GraphPad Prism software (version 9.0.0). 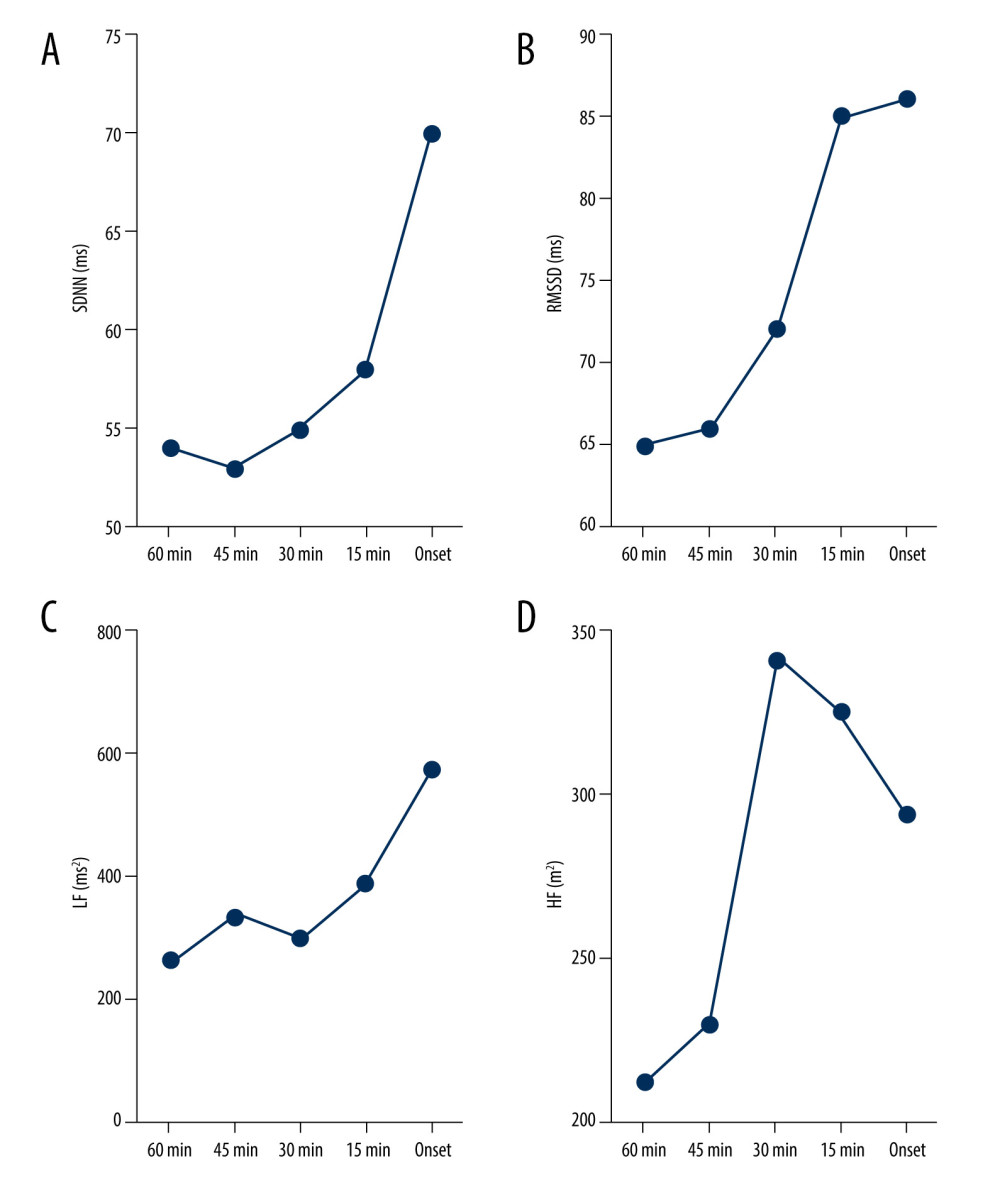 Figure 3. Dynamic variations in SDNN (A), RMSSD (B), LF (C), and HF (D) before sympathetic PAF onset. Details were described in the text. The figures were created using GraphPad Prism software (version 9.0.0).
Figure 3. Dynamic variations in SDNN (A), RMSSD (B), LF (C), and HF (D) before sympathetic PAF onset. Details were described in the text. The figures were created using GraphPad Prism software (version 9.0.0). 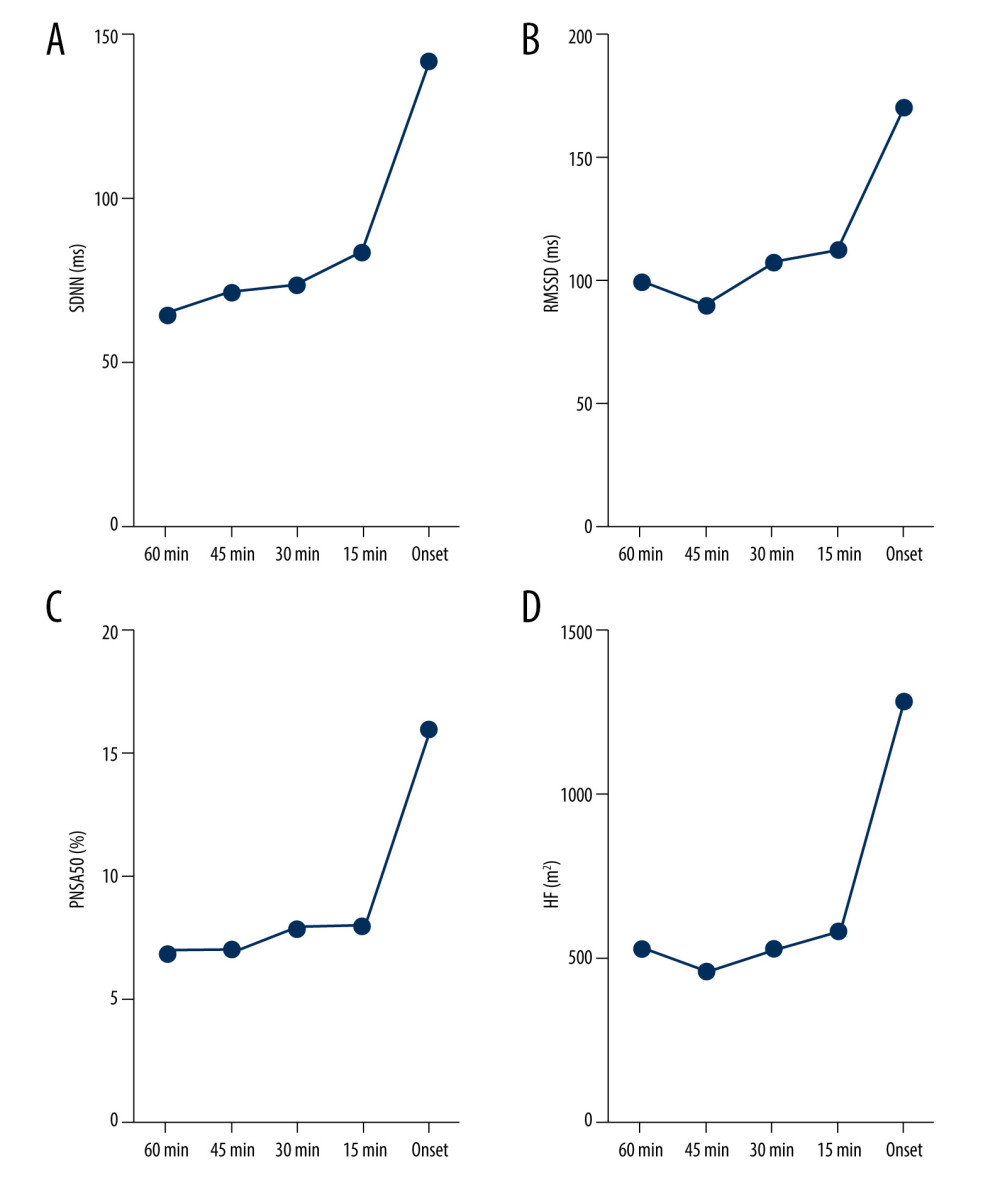 Figure 4. Dynamic variations in SDNN (A), RMSSD (B), PNN50 (C), and HF (D) before vagus AF onset. Details were described in the text. The figures were created using GraphPad Prism software (version 9.0.0).
Figure 4. Dynamic variations in SDNN (A), RMSSD (B), PNN50 (C), and HF (D) before vagus AF onset. Details were described in the text. The figures were created using GraphPad Prism software (version 9.0.0). 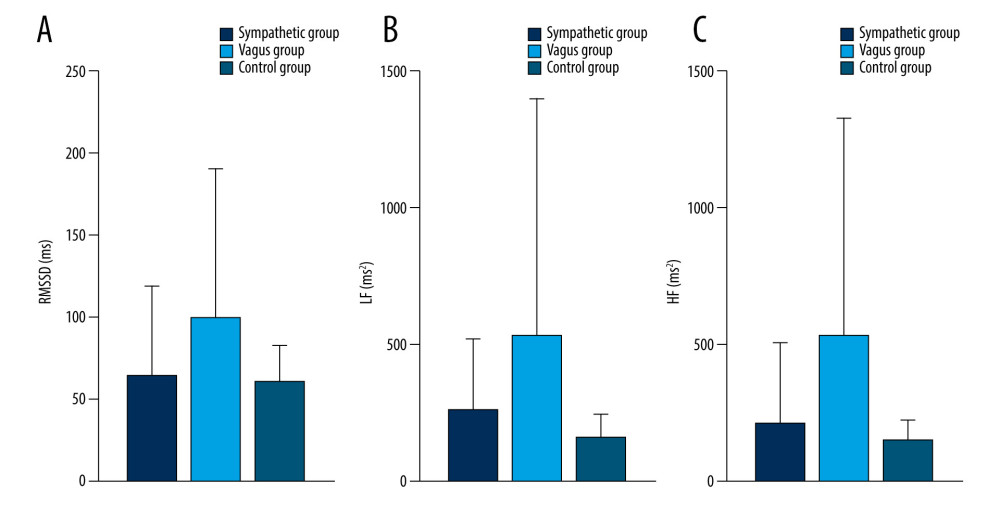 Figure 5. Dynamic variations of PAF group and control group in RMSSD (A), LF (B) and HF (C) during 60 minutes before PAF. Details were described in the text. The figures were created using GraphPad Prism software (version 9.0.0).
Figure 5. Dynamic variations of PAF group and control group in RMSSD (A), LF (B) and HF (C) during 60 minutes before PAF. Details were described in the text. The figures were created using GraphPad Prism software (version 9.0.0). 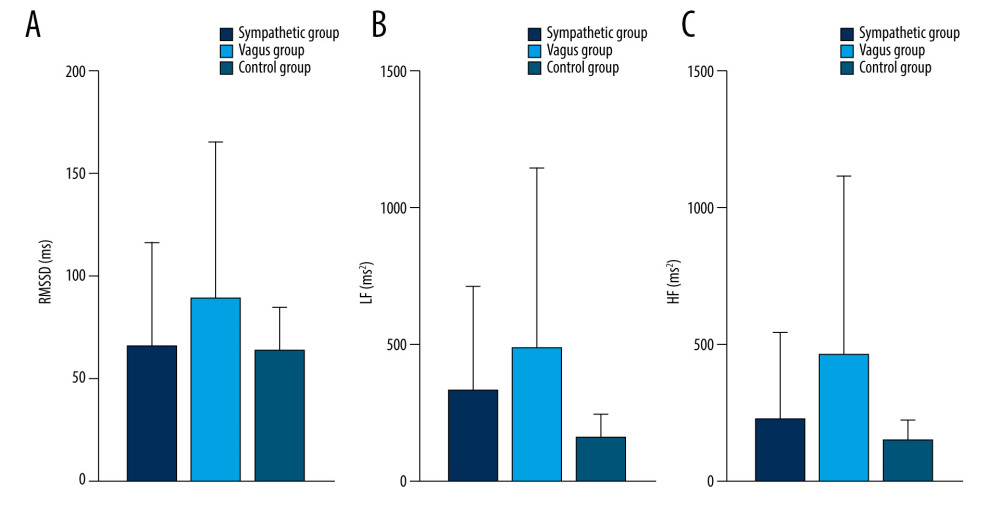 Figure 6. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), and HF (C) during 45 minutes before PAF. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0).
Figure 6. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), and HF (C) during 45 minutes before PAF. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0). 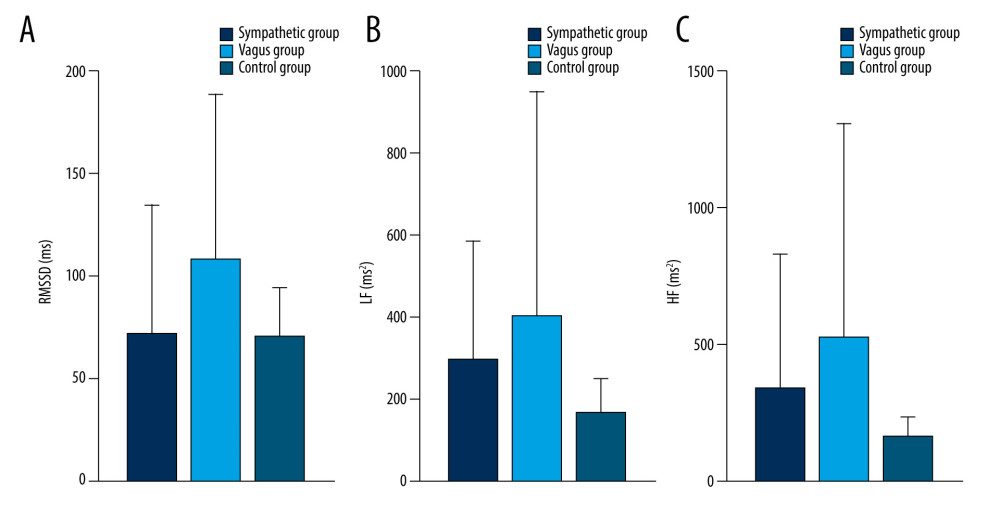 Figure 7. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), and HF (C) during 30 minutes before PAF. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0).
Figure 7. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), and HF (C) during 30 minutes before PAF. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0). 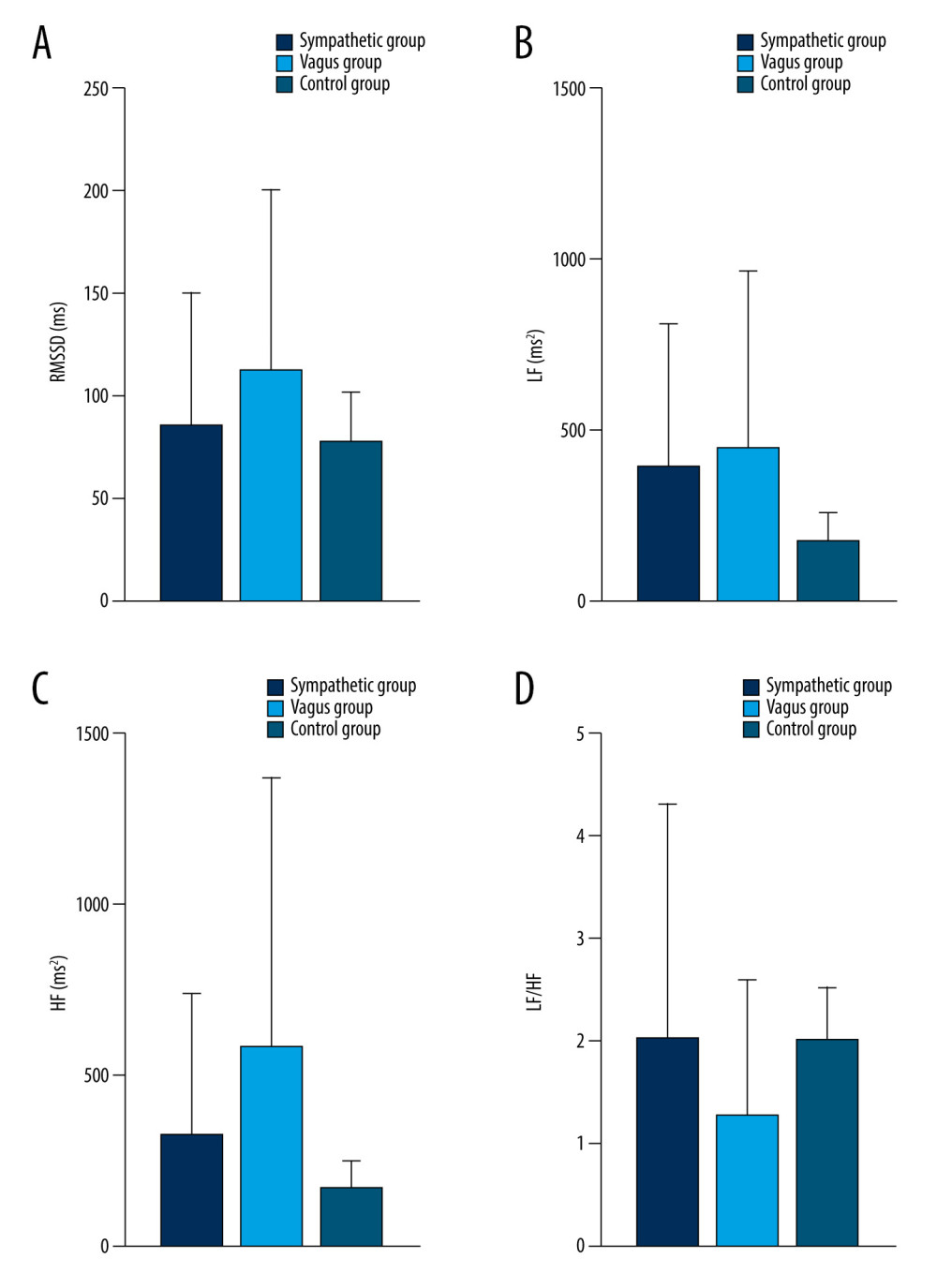 Figure 8. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), HF (C), and LF/HF (D) during 15 minutes before PAF. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0).
Figure 8. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), HF (C), and LF/HF (D) during 15 minutes before PAF. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0). 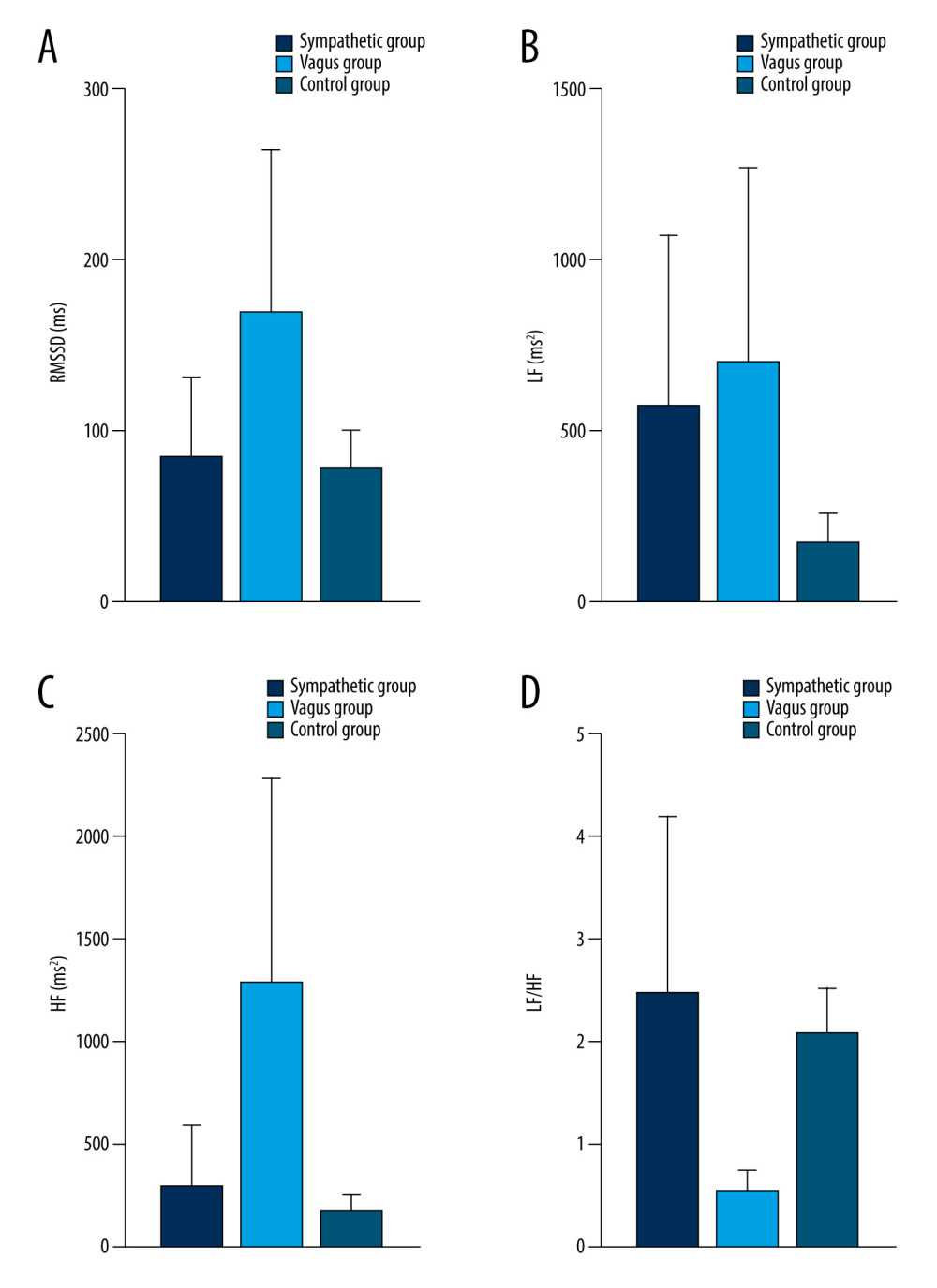 Figure 9. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), HF (C), and LF/HF (D) at PAF onset. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0).
Figure 9. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), HF (C), and LF/HF (D) at PAF onset. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0). Tables
Table 1. Time and frequency domain parameters.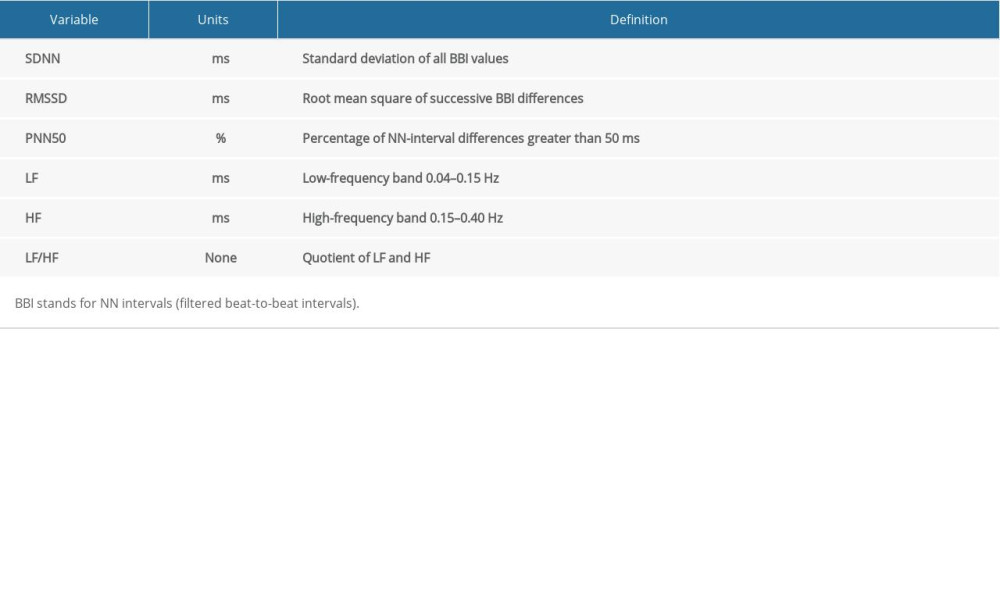 Table 2. Baseline characteristics of study population.
Table 2. Baseline characteristics of study population.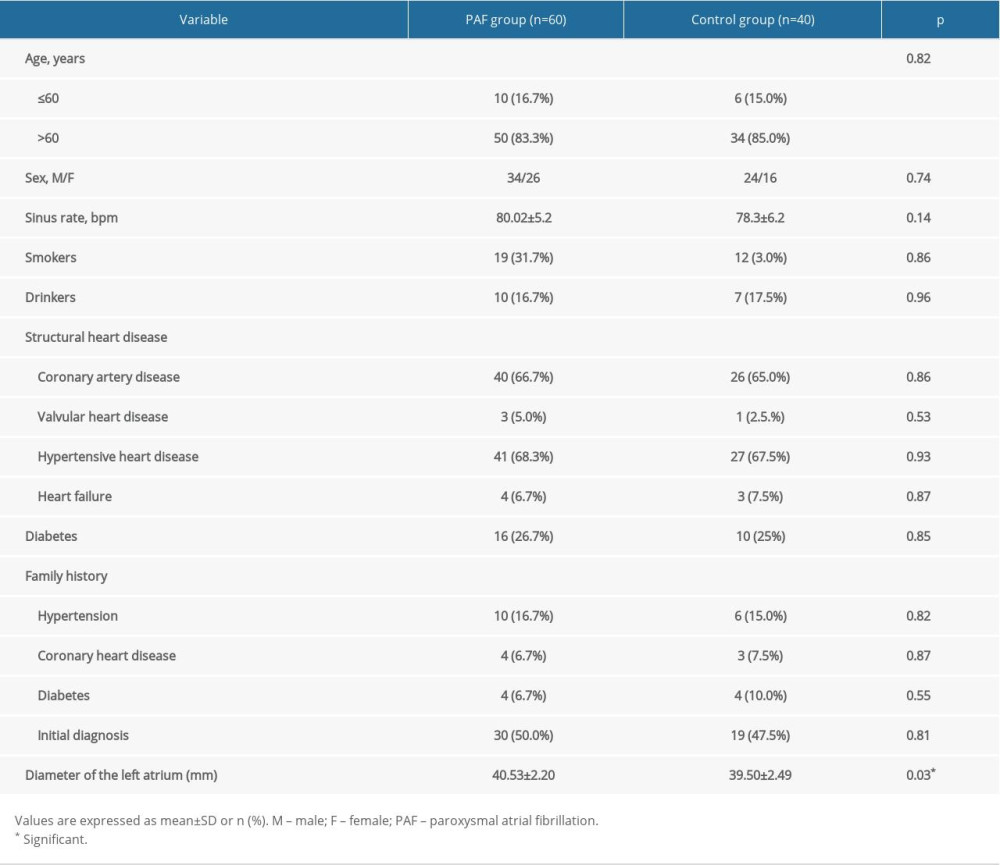 Table 3. Variations in HRV parameters before PAF onset in sympathetic group.
Table 3. Variations in HRV parameters before PAF onset in sympathetic group.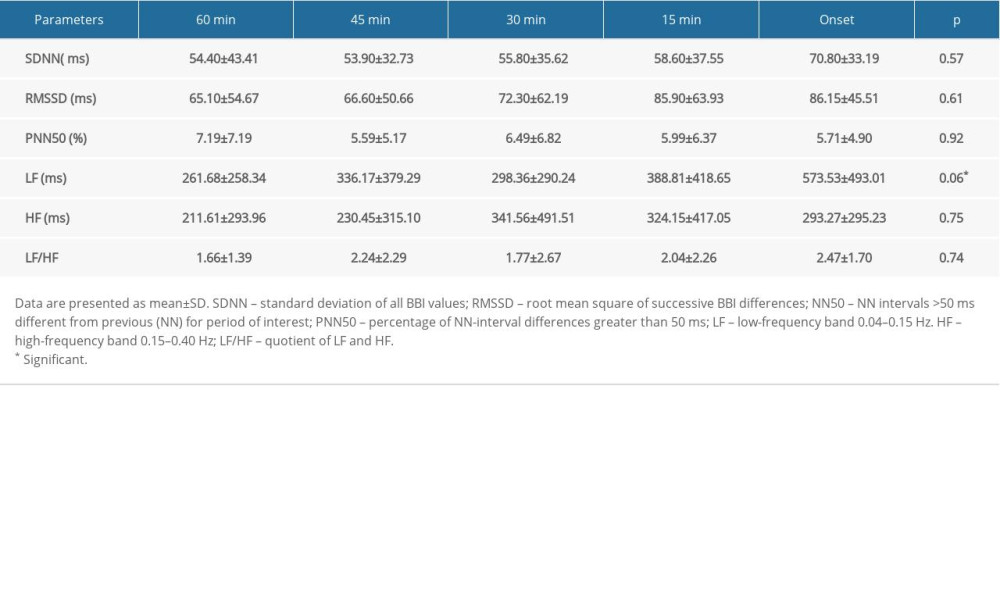 Table 4. Variations in HRV parameters before PAF onset in vagus group.
Table 4. Variations in HRV parameters before PAF onset in vagus group.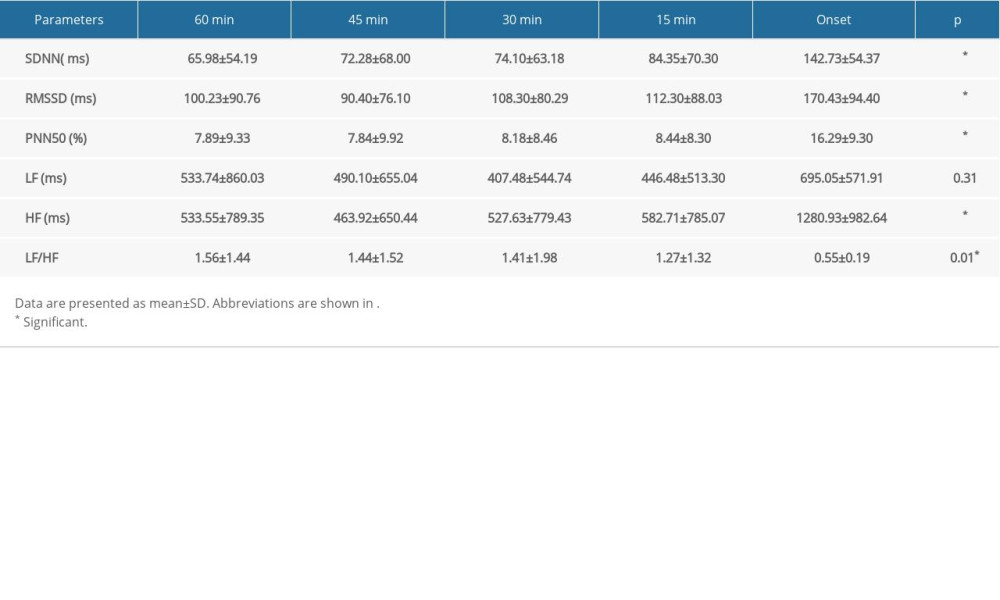 Table 5. Comparison of HRV parameters between patients with PAF and controls.
Table 5. Comparison of HRV parameters between patients with PAF and controls.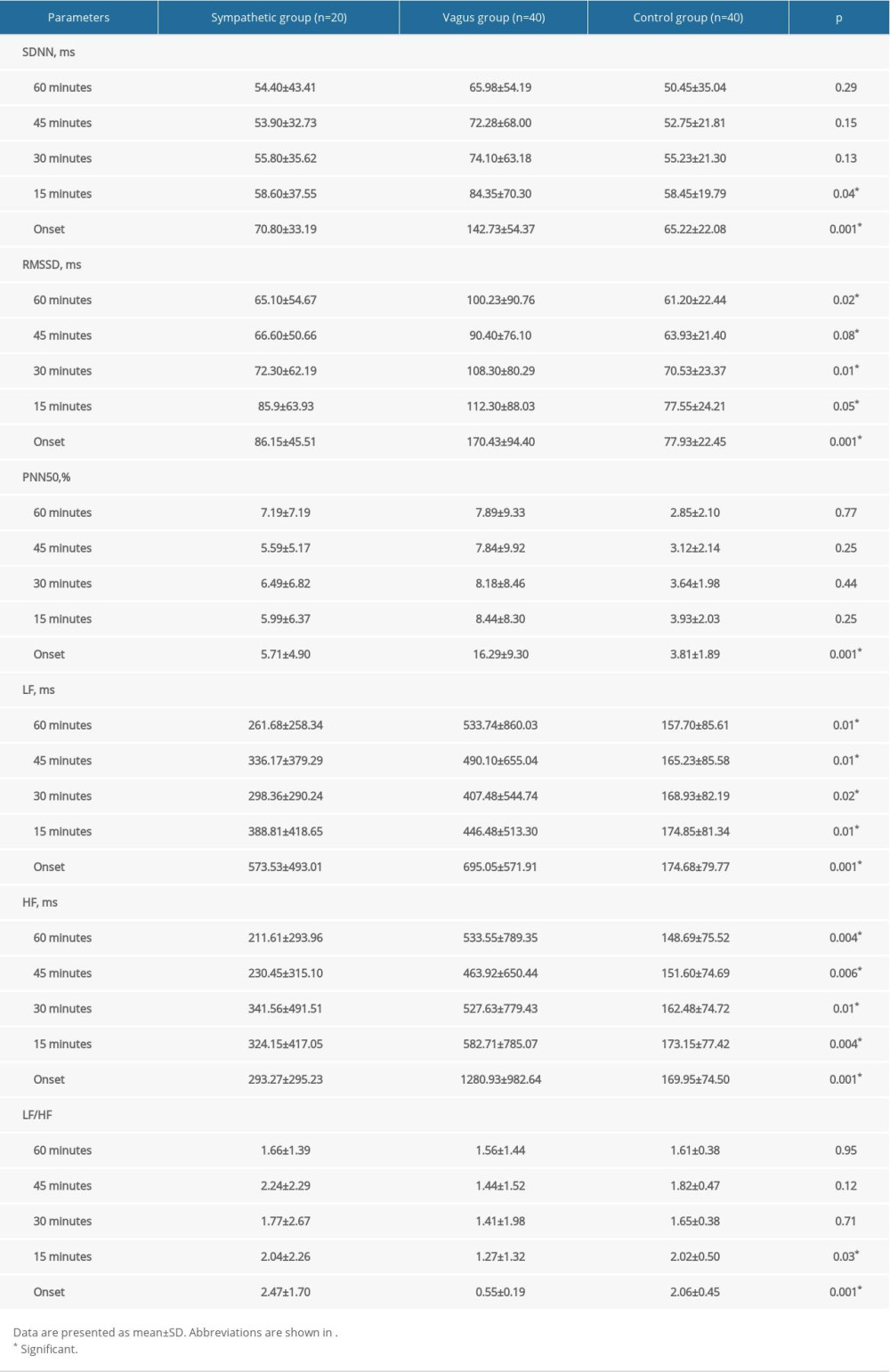
References
1. Chugh SS, Havmoeller R, Narayanan K, Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study: Circulation, 2014; 129(8); 837-47
2. Ganesan AN, Shipp NJ, Brooks AG, Long-term outcomes of catheter ablation of atrial fibrillation: A systematic review and meta-analysis: J Am Heart Assoc, 2013; 2(2); e004549
3. Weerasooriya R, Khairy P, Litalien J, Catheter ablation for atrial fibrillation: Are results maintained at 5 years of follow-up?: J Am Coll Cardiol, 2011; 57; 160-66
4. Gallo C, Bocchino PP, Magnano M, Autonomic tone activity before the onset of atrial fibrillation: J Cardiovasc Electrophysiol, 2017; 28(3); 304-14
5. Chen PS, Chen LS, Fishbein MC, Role of the autonomic nervous system in atrial fibrillation: Pathophysiology and therapy: Circ Res, 2014; 114(9); 1500-15
6. Chung MK, Refaat M, Shen WK, Atrial fibrillation: JACC council perspectives: J Am Coll Cardiol, 2020; 75(14); 1689-713
7. Tan AY, Li H, Wachsmann-Hogiu S, Chen LS, Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: Implications for catheter ablation of atrial-pulmonary vein junction: J Am Coll Cardiol, 2006; 48; 132-43
8. Qin M, Zeng C, Liu X, The cardiac autonomic nervous system: A target for modulation of atrial fibrillation: Clin Cardiol, 2019; 42(6); 644-52
9. Khan AA, Lip GYH, Shantsila A, Heart rate variability in atrial fibrillation: The balance between sympathetic and parasympathetic nervous system: Eur J Clin Invest, 2019; 49(11); e13174
10. Rajendra Acharya U, Paul Joseph K, Kannathal N, Heart rate variability: A review: Med Biol Eng Comput, 2006; 44(12); 1031-51
11. Saul JP, Beat-to-beat variations of heart rate reflect modulation of cardiac autonomic outflow: News Physiol Sci, 1990; 5; 32-37
12. Barauskiene V, Rumbinaite E, Karuzas A, Importance of heart rate variability in patients with atrial fibrillation: J Cardiol Clin Res, 2016; 4; 1080
13. Huang JL, Wen ZC, Lee WL, Changes of autonomic tone before the onset of paroxysmal atrial fibrillation: Int J Cardiol, 1998; 66; 275-83
14. Fioranelli M, Piccoli M, Mileto GM, Analysis of heart rate variability five minutes before the onset of paroxysmal atrial fibrillation: Pacing Clin Electrophysiol, 1999; 22(5); 743-49
15. Lombardi F, Tarricone , Tundo F, Autonomic nervous system and paroxysmal atrial fibrillation: A study based on the analysis of RR interval changes before, during and after paroxysmal atrial fibrillation: Eur Heart J, 2004; 25; 1242-48
16. Sammito S, Böckelmann I, Reference values for time- and frequency-domain heart rate variability measures: Heart Rhythm, 2016; 13(6); 1309-16
17. Nattel S, Dobrev D, Electrophysiological and molecular mechanisms of paroxysmal atrial fibrillation: Nat Rev Cardiol, 2016; 13(10); 575-90
18. Calkins H, Hindricks G, Cappato R, 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Europace, 2018; 20(1); e1-160
19. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, Heart rate variability: Standards of measurement, physiological interpretation, and clinical use: Circulation, 1996; 93; 1043-65
20. Wessel N, Berg K, Kraemer JF, Cardiac autonomic dysfunction and incidence of de novo atrial fibrillation: Heart rate variability vs. heart rate complexity: Front Physiol, 2020; 11; 596844
21. Jiang LZ, 2015, Fujian Medical University Available from: URL: [in Chinese]https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201601&filename=1015991424.nh&v=%25mmd2FjzKxMvsJpRT7VthP2TZ7fpAdAM5kuk2d7ICymVgp5OQsVay6%25mmd2BHjFa3lDhJ0zM5H
22. Baye’s de Luna A, Baye’s Genis A, Guindo J, Mechanisms favoring and triggering atrial fibrillation: Arch Mal Coeur, 1994; 87; 19-25
23. Smeets JLRM, Allessie MA, Lammers WJEP, The wavelength of the cardiac impulse and reentrant arrhythmias in isolated rabbit atrium: The role of the heart rate, autonomic transmitters, temperature, and potassium: Circ Res, 1986; 58; 96-108
24. Ninomyia I, Direct evidence of nonuniform distribution of vagal effects on dog atria: Circ Res, 1966; 19; 576-83
25. Francis GS, Modulation of peripheral sympathetic nerve transmission: J Am Coll Cardiol, 1988; 12; 250-54
26. van den Berg MP, Haaksma J, Brouwer J, Heart rate variability in patients with atrial fibrillation is related to vagal tone: Circulation, 1997; 96(4); 1209-16
27. Chou CC, Chen PS, New concepts in atrial fibrillation: Neural mechanisms and calcium dynamics: Cardiol Clin, 2009; 27(1); 35-43
28. Bettoni M, Zimmermann M, Autonomic tone variations before the onset of paroxysmal atrial fibrillation: Circulation, 2002; 105(23); 2753-59
29. Fioranelli M, Piccoli M, Mileto GM, Analysis of heart rate variability five minutes before the onset of paroxysmal atrial fibrillation: Pacing Clin Electrophysiol, 1999; 22(5); 743-49
30. Coumel P, Paroxysmal atrial fibrillation: a disorder of autonomic tone?: Eur Heart J, 1994; 15(Suppl A); 9-16
31. Platiša MM, Bojić T, Pavlović SU, Generalized poincaré plots – a new method for evaluation of regimes in cardiac neural control in atrial fibrillation and healthy subjects: Front Neurosci, 2016; 10; 38
32. Zuo MPoincaré scatter plot on the diagnosis of autonomic nerve types in atrial fibrillation, 2012, Xinjiang Medical University Available from: URL: [in Chinese]https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201301&filename=1012489397.nh&v=wPnxG9vczLqCa6afbF1%25mmd2FhwssRpfZ6FXD7AiQPHNQx0kIT9%25mmd2FpBRbUj7cNHWo58BC8
33. Garcia-Isla G, Corino V, Mainardi L, Poincaré plot image and rhythm-specific atlas for atrial bigeminy and atrial fibrillation detection: IEEE J Biomed Health Inform, 2021; 25(4); 1093-100
34. Shaffer F, Ginsberg JP, An overview of heart rate variability metrics and norms: Front Public Health, 2017; 5; 258
35. Shaffer F, McCraty R, Zerr CL, A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability: Front Psychol, 2014; 5; 1040
36. Tiller WA, McCraty R, Atkinson M, Cardiac coherence: A new, noninvasive measure of autonomic nervous system order: Altern Ther Health Med, 1996; 2; 52-65
37. Kleiger RE, Stein PK, Bigger JT, Heart rate variability: Measurement and clinical utility: Ann Noninvasive Electrocardiol, 2005; 10(1); 88-101
38. McCraty R, Shaffer F, Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk: Glob Adv Health Med, 2015; 4; 46-61
Figures
 Figure 1. Poincaré dispersed-dot plot of PAF. (A) Oval shape, (B) spider shape, (C) irregular shape, (D) comet shape.
Figure 1. Poincaré dispersed-dot plot of PAF. (A) Oval shape, (B) spider shape, (C) irregular shape, (D) comet shape. Figure 2. Distribution of different shapes of Poincaré dispersed-dot plot for PAF group. The figure was created using GraphPad Prism software (version 9.0.0).
Figure 2. Distribution of different shapes of Poincaré dispersed-dot plot for PAF group. The figure was created using GraphPad Prism software (version 9.0.0). Figure 3. Dynamic variations in SDNN (A), RMSSD (B), LF (C), and HF (D) before sympathetic PAF onset. Details were described in the text. The figures were created using GraphPad Prism software (version 9.0.0).
Figure 3. Dynamic variations in SDNN (A), RMSSD (B), LF (C), and HF (D) before sympathetic PAF onset. Details were described in the text. The figures were created using GraphPad Prism software (version 9.0.0). Figure 4. Dynamic variations in SDNN (A), RMSSD (B), PNN50 (C), and HF (D) before vagus AF onset. Details were described in the text. The figures were created using GraphPad Prism software (version 9.0.0).
Figure 4. Dynamic variations in SDNN (A), RMSSD (B), PNN50 (C), and HF (D) before vagus AF onset. Details were described in the text. The figures were created using GraphPad Prism software (version 9.0.0). Figure 5. Dynamic variations of PAF group and control group in RMSSD (A), LF (B) and HF (C) during 60 minutes before PAF. Details were described in the text. The figures were created using GraphPad Prism software (version 9.0.0).
Figure 5. Dynamic variations of PAF group and control group in RMSSD (A), LF (B) and HF (C) during 60 minutes before PAF. Details were described in the text. The figures were created using GraphPad Prism software (version 9.0.0). Figure 6. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), and HF (C) during 45 minutes before PAF. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0).
Figure 6. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), and HF (C) during 45 minutes before PAF. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0). Figure 7. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), and HF (C) during 30 minutes before PAF. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0).
Figure 7. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), and HF (C) during 30 minutes before PAF. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0). Figure 8. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), HF (C), and LF/HF (D) during 15 minutes before PAF. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0).
Figure 8. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), HF (C), and LF/HF (D) during 15 minutes before PAF. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0). Figure 9. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), HF (C), and LF/HF (D) at PAF onset. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0).
Figure 9. Dynamic variations of PAF group and control group in RMSSD (A), LF (B), HF (C), and LF/HF (D) at PAF onset. Details were described in the text. Data are presented as mean±SD. The figures were created using GraphPad Prism software (version 9.0.0). Tables
 Table 1. Time and frequency domain parameters.
Table 1. Time and frequency domain parameters. Table 2. Baseline characteristics of study population.
Table 2. Baseline characteristics of study population. Table 3. Variations in HRV parameters before PAF onset in sympathetic group.
Table 3. Variations in HRV parameters before PAF onset in sympathetic group. Table 4. Variations in HRV parameters before PAF onset in vagus group.
Table 4. Variations in HRV parameters before PAF onset in vagus group. Table 5. Comparison of HRV parameters between patients with PAF and controls.
Table 5. Comparison of HRV parameters between patients with PAF and controls. Table 1. Time and frequency domain parameters.
Table 1. Time and frequency domain parameters. Table 2. Baseline characteristics of study population.
Table 2. Baseline characteristics of study population. Table 3. Variations in HRV parameters before PAF onset in sympathetic group.
Table 3. Variations in HRV parameters before PAF onset in sympathetic group. Table 4. Variations in HRV parameters before PAF onset in vagus group.
Table 4. Variations in HRV parameters before PAF onset in vagus group. Table 5. Comparison of HRV parameters between patients with PAF and controls.
Table 5. Comparison of HRV parameters between patients with PAF and controls. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








