24 March 2022: Clinical Research
Efficacy of PD-1 Inhibitor Combined with Bevacizumab in Treatment of Advanced Endometrial Cancer Patients with Mismatch Repair Deficiency (dMMR)/High-Level Microsatellite Instability (MSI-H)
Ying Liao1BCE, Changhua ZhuDOI: 10.12659/MSM.934493
Med Sci Monit 2022; 28:e934493
Abstract
BACKGROUND: Mismatch repair deficiency (dMMR) is associated with endometrial cancers, yet it remains unknown how this information could be incorporated into adjuvant treatment paradigms. We performed this cohort study to identify the effect of dMMR status on the prognosis of patients with advanced endometrial cancer treated with PD-1 inhibitor and bevacizumab.
MATERIAL AND METHODS: We enrolled 93 patients with advanced endometrial cancer and divided them into an observation group (n=52) and a control group (n=41) according to the treatment. The control group was treated with bevacizumab combined with paclitaxel chemotherapy, while the observation group was treated with PD-1inhibitor combined with bevacizumab. The basic characteristics and overall survival times were compared between the 2 groups.
RESULTS: There was no significant difference in age, course of disease, clinical stage, or pathological type. The proportion of patients with dMMR and high-level microsatellite instability (MSI-H) were balanced in the 2 groups. Patients in the observation group had longer overall survival than those in the control group (33.2 months vs 21.8 months). Moreover, in the observation group, the median OS of dMMR patients was not detected, while the median OS of PMMR patients was 29.2 months (P<0.01). In the control group, the median OS of dMMR patients was 12.4 months, and that of PMMR patients was 24.1 months (P<0.01).
CONCLUSIONS: Advanced endometrial cancer patients with dMMR/MSI-H treated with PD-1 inhibitor plus bevacizumab had longer overall survival (OS) than those treated with bevacizumab plus paclitaxel chemotherapy.
Keywords: bevacizumab, Endometrial Neoplasms, PDCD1 Protein, Human, Brain Neoplasms, Cohort Studies, Colorectal Neoplasms, DNA Mismatch Repair, Female, Humans, Immune Checkpoint Inhibitors, microsatellite instability, Neoplastic Syndromes, Hereditary
Background
Endometrial carcinoma (EC) is a group of primary epithelial malignant tumors in the endometrium. It is one of the 3 major gynecological malignancies, with the highest mortality rate [1]. International Federation of Gynecology and Obstetrics (FIGO) staging reflects the 5-year survival rate, and the majority of patients have favorable outcomes because of early-stage disease at diagnosis [2]. However, patients with advanced disease have poor outcomes, with 5-year survival rates of about 45% and 25% for stages III and IV, respectively [3]. The DNA mismatch repair (MMR) system is necessary for the maintenance of genomic stability. Evaluation of endometrial tumors for MMR deficiency can identify patients with Lynch syndrome, an autosomal dominant mutation in the DNA of MMR proteins, including mutL homolog 1(MLH1), mutS homolog 2 (MSH2), mutS homolog 6 (MSH6), or PMS1 homolog 2 (PMS2). Approximately 3% of endometrial cancers are attributable to Lynch syndrome [4]. MMR deficiency is typically evaluated via immunohistochemistry (IHC) for MMR protein expression and polymerase chain reaction assessment of DNA microsatellite instability (MSI) [5].
At present, the treatment of endometrial cancer mainly includes surgery, chemoradiotherapy, and other treatments [6]. Immunotherapy with programmed death 1(PD-1) inhibitors has shown good efficacy in cervical cancer and other malignant tumors [7]. A foreign study showed that 26% of patients with PD-L1-positive advanced recurrent endometrial carcinoma achieved remission or stabilization after treatment with PD-1 inhibitor [9]. Subsequently, Le et al [10] conducted a study on multiple solid tumors with MSI, and found that tumors with MSI had a better response to immunotherapy with PD-1 inhibitors, and the effective rate was 53% in patients with endometrial cancer. In 2017, the U.S. Food and Drug Administration accelerated the approval of pembrolizumab, a PD-1 inhibitor, for the treatment of dMMR/MSI-H advanced solid tumors based on the research of Le et al.
Rose et al [11,12] demonstrated that the combination of bevacizumab, paclitaxel, and carboplatin is active and tolerable in a large cohort of patients with advanced endometrial carcinoma. In addition, many clinical studies on the treatment of endometrial cancer with PD-1 inhibitors alone or in combination with hormones and small-molecule drugs are also underway. Thus, the present study mainly assessed the effect of dMMR status on the prognosis of patients with advanced endometrial cancer treated with PD-1 inhibitor and bevacizumab.
Material and Methods
PATIENTS:
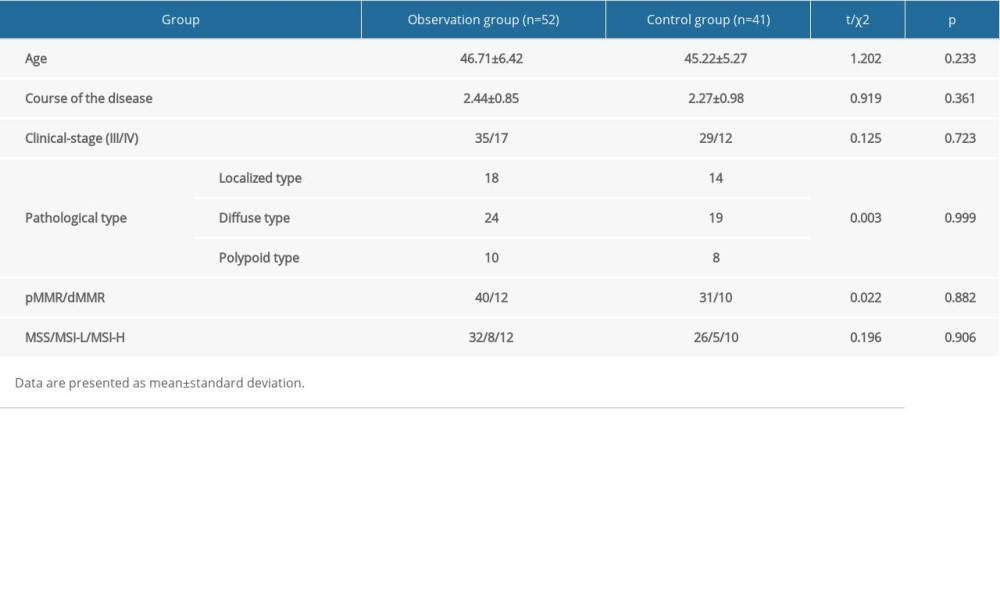
In this study,93patients with advanced endometrial cancer treated in our hospital from January 2017 to June 2018 were enrolled. All patients were diagnosed with advanced endometrial cancer confirmed by histological analysis and MRI. The study was conducted in accordance with the Declaration of Helsinki. Patients provided written informed consent. The study was approved by the Ethics Committee of the XinYu People’s Hospital. We excluded patients with liver and kidney dysfunction or abnormalities, as well as those with history of allergy to drugs such as paclitaxel, carboplatin, and bevacizumab. The patient selection process is depicted schematically in Figure 1.
MRI EXAMINATION:
All patients were examined using a GE 1.5T superconducting MRI scanner with an 8-channel phased array coil. Before the examination, patients were instructed to keep a full bladder and placed in supine position for a routine plain scan. The scan sequences included transection, coronal planes, sagittal plane autogyro wave T2W1 and T2W1 fat suppression sequence, and transection autogyro wave T1W1 sequence. After plain scanning, enhanced scanning was performed. The contrast agent gadolinium spray meglumine (0.1 mmol/kg) was injected intravenously to analyze tumor morphology, size, echo, and signal intensity under different sequences of scanning.
TREATMENT METHODS:
According to the treatment, patients were divided into 2 groups: PD-1 inhibitor (nivolumab) combined with bevacizumab was the observation group (n=52) and bevacizumab combined with paclitaxel chemotherapy was the control group (n=41). Drug dose and cycle were given according to the instructions. The observation group patients were treated with bevacizumab plus nivolumab every 2 weeks for 8 cycles followed by maintenance with bevacizumab plus nivolumab every 2 weeks until disease progression, unacceptable toxicity, or patient/physician decision. Bevacizumab was administered intravenously at a dose of5 mg/kg every 2 weeks. Nivolumab was administered intravenously at a flat dose of 240 mg every 2 weeks. The control group patients were treated with bevacizumab plus paclitaxel chemotherapy. Paclitaxel 175 mg/m2 over 3 h and bevacizumab at 15 mg/kg were administered every 21 days.
IMMUNOHISTOCHEMICAL METHODS FOR MMR PROTEIN:
IHC evaluation of MMR protein expression was performed on full sections. Endometrial tissue samples, which had been stored as paraffin wax-embedded tissue blocks, were deparaffinized in xylene and rehydrated in graded ethanol. Then, samples were incubated with primary antibodies of MLH1, MSH2, MSH6, and PMS2 (cat. no. ab252190, Abcam). Immunostaining was performed according to previously described protocols [13]. Briefly, 4-mm sections were cut from the tissue and mounted on aminopropyl-ethoxy-silane-coated glass slides. Sections were deparaffinized in xylene and rehydrated in ethanol. Endogenous peroxidase was blocked by incubation in a 0.3% H2O2 solution for 30 min. After dewaxing and antigen extraction, the sections were incubated with antibodies, developed by DAB solution, counterstained with hematoxylin, sealed, and photographed. Each section was observed from 5 fields with a microscope. The primary antibody and secondary antibody were MLH1 (ab92312, Abcam), MSH2 (ab227941), MSH6 (ab92471), PMS2 (ab110638), and Anti-Rabbit IgG (ab205718). Evaluation of the MMR protein expression status was performed by2 pathologists. Cases with complete absence of nuclear staining and positive nuclear staining in internal control cells were considered to demonstrate MMR protein loss. A tumor was considered aberrant if tumor cells showed complete absence of nuclear staining with positive non-neoplastic internal control, and were considered normal if tumor cells showed nuclear positivity.
MULTIPLEX PCR (HIGH-RESOLUTION CAPILLARY ELECTROPHORESIS [HRCE]):
We extracted DNA from tumorous and adjacent non-tumorous tissue. We assessed changes in fragment lengths both for mono-nucleotide and di-nucleotide markers (BAT25, BAT26 D2S123, D5S346, and D17S250, respectively) using multiplex PCR combined with HRCE. MSI-H was defined if ≥2 out of 5 markers were found to be unstable.
For confirmation of MSI, tissue samples of all patients were tested by capillary electrophoresis and the measurement was performed by Shanghai Tech Labway Co., Ltd (Shanghai, China) (Figure 2). Briefly, microsatellite instability analysis was performed in all tumorous and non-tumorous samples. MSI was detected using the Promega MSI Analysis System (Version 2.0) according to the manufacturer’s protocol (Promega). The MSI Analysis System included fluorescently labeled primers for the coamplification of 5 markers, including mono-nucleotide and di-nucleotide markers (BAT25, BAT26 D2S123, D5S346, and D17S250). The PCR products were separated using an ABI 3500DX Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA, USA). Then, the output data were analyzed with GeneMapper® Analysis Software (Thermo Fisher Scientific) to identify MSI status. The results were classified as high microsatellite instability (MSI-H) (microsatellite alterations in at least 2 of the 5 markers), low microsatellite instability (MSI-L) (microsatellite alteration in one marker), and microsatellite stable (MSS) (no microsatellite alteration) according to the National Cancer Institute (NCI) criteria and the manufacture’s protocol (Promega, Madison, WI, USA).
STATISTICAL ANALYSIS:
Patients’ clinical data were retrieved from hospital patient files. All values are expressed as the mean±standard error of the mean (SEM) and as percentages. Analysis of variance (ANOVA) and chi-squared test were performed to compare the age, course of the disease, clinical stage, and pathological type. Kaplan-Meier survival curves were compared using the log-rank test. Two-sided
Results
BASIC CHARACTERISTICS OF ALL PATIENTS:
Of the 93 patients, 52 received PD-1 inhibitor combined with bevacizumab immunotherapy, and 41 received bevacizumab combined with paclitaxel chemotherapy. All patients were diagnosed with advanced endometrial cancer and were confirmed by both histological analysis and MRI (Figures 3, 4). The clinical characteristics of the 2 groups are shown in Table 1. There was no significant difference in age, course of disease, clinical stage, or pathological type. The proportion of patients with dMMR was balanced in the 2 groups. As shown in Figure 5, the deletion of MMR proteins was detected by immunohistochemistry staining. In addition, the ratio of patients with MSI-H in the 2 groups was similar (12/52 vs 10/41).
PROGNOSIS ANALYSIS:
The median OS was 29.2 months (95% CI 27.4–30.9 months). The median OS of the observation group was 33.2 months (95% CI 26.7–39.6 months) and that of the control group was 21.8 months (95% CI 17.9–25.7 months), as shown in Figure 6. Patients in the observation group had longer OS than those in the control group.
As shown in Figure 7, in the observation group, the median OS of dMMR patients was not detected, while the median OS of PMMR patients was 29.2 months (P=0.0002). In the control group, the median OS of dMMR patients was 12.4 months, and that of PMMR patients was 24.1 months (P<0.0001). These data suggest that the effects of different treatments varied significantly according to mismatch repair status.
Discussion
Endometrial carcinoma is a tumor with great heterogeneity in biological, molecular, and pathological aspects. The limitations of traditional hormone-dependent classification and WHO pathological type have been reported [14]. In 2013, The Cancer Genome Atlas (TCGA)classified endometrial cancer into 4 types based on the molecular level: DNA polymerization mutation, MSI type, low copy number type, and high copy number type [15]. In 2015, the improved TCGA classification system was revised. Molecular typing of endometrial cancer can help develop individualized patient treatment. MSI accounted for 25–30% of dMMR/MSI-H types in endometrial carcinoma. In this study, MSI-H/dMMR was found in 23.65% (22/93) of advanced endometrial cancer patients.
With the development of endometrial carcinoma, it is found that the degree of EC proliferation and metastasis is positively correlated with tumor blood vessels. Now, researchers are trying to use anti-vascular endothelial growth factor targeted drug delivery to inhibit the growth of tumor blood vessels, which causes tumor growth inhibition. As the first anti-angiogenesis monoclonal antibody drug approved for use in metastatic colorectal cancer, breast cancer, and non-small cell lung cancer in the United States, bevacizumab has attracted academic attention. Viswanathan et al [16] reported that 15 patients with recurrent endometrial cancer received radiotherapy combined with bevacizumab, with 1- and 3-year PFS of 80% and 67%, respectively and OS 93% and 80%, respectively, which suggested that this scheme can be used in patients with recurrent endometrial cancer in the future. Clinical studies with approved indications show that the incidence of thromboembolism, gastrointestinal fistula, perforation, hypertension, and other complications of single-drug chemotherapy is lower than that of conventional single-drug chemotherapy when combined with bevacizumab, and the severity of adverse reactions is slightly lower than that of single-drug chemotherapy [17]. According to GOG, the prognosis of recurrent and metastatic endometrial carcinoma is often poor. The standard chemotherapy regimen is based on platinum combined with cytotoxic drugs such as paclitaxel. The incidence of adverse effects is high, and 46% of patients have neurotoxicity of grade 2 or above [18]. Chemotherapy combined with bevacizumab is generally well tolerated, with little toxicity. This study found that the median OS of patients receiving bevacizumab combined with paclitaxel chemotherapy was 21.8 months (95% CI 17.9–25.7 months).
PD-1 is expressed on the surface of T cells and primary B cells and plays a role in the differentiation and apoptosis of these cells. PD-L1 protein is widely expressed in antigen-presenting cells (APCs), activated T cells, activated B cells, macrophages, placental trophoblast cells, myocardial endothelial cells, and thymic cortical epithelial cells. PD-1 binds to PD-L1, a ligand expressed in tumors, and inhibits T cells, thus inhibiting the anti-tumor immune response. PD-1 antibody and PD-L1 antibody did not kill cancer cells, but activated T cells by blocking PD-1/PD-L1 mediated inhibitory signal transmission, and triggered the anti-tumor effect of the patient’s autoimmune system [19]. In early clinical trials, PD-1 antibody and PD-L1 antibody have shown good curative effects on lung cancer, gastric cancer, melanoma, and other types of tumors, and the clinical adverse effects can be controlled by steroid drugs [20]. PD-1 inhibitors have a broad anticancer spectrum, few adverse effects, and long-term efficacy, which bring hope for a clinical cure to many patients with advanced cancer [21]. In the present study, patients in the PD-1 inhibitor combined with bevacizumab group had longer overall survival (33.2 months, 95% CI 26.7–39.6 months) than those in the bevacizumab combined with paclitaxel chemotherapy group, demonstrating the efficacy of PD-1 inhibitor combined with bevacizumab in the treatment of advanced endometrial cancer.
In addition, TSR-042, a new anti-human PD-1 therapeutic antibody, is in phase I clinical trial stage for the treatment of endometrial cancer. According to the trial data published in October 2018, 13 out of 25 patients with MSI-H had complete or partial remission [22]. In the present study, the median OS of dMMR patients was not assessed, while the median OS of PMMR patients was 29.2 months in the PD-1 inhibitor combined with the bevacizumab group. But the median OS of dMMR patients was 12.4 months, and that of PMMR patients was 24.1 months, suggesting that the effects of different treatments varied significantly according to MMR status.
Conclusions
The present study found that the OS of patients with advanced endometrial cancer with dMMR/MSI-H receiving PD-1 inhibitor combined with bevacizumab was better than that of patients receiving bevacizumab combined with paclitaxel chemotherapy. One limitation of this study is the small number of enrolled patients. Future studies should recruit a large number of patients to participate.
Figures
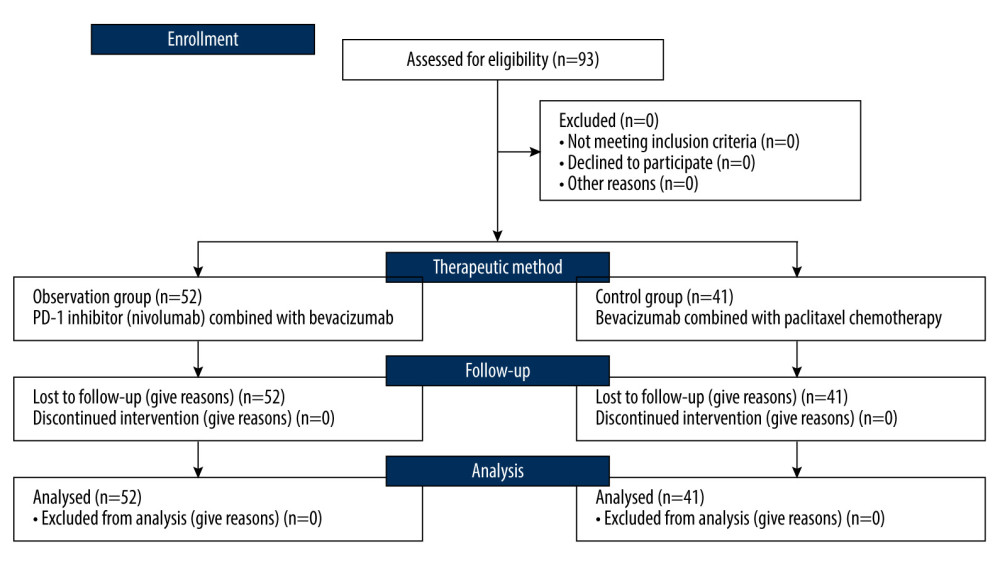 Figure 1. Flow diagram demonstrating the patient selection process in this study. The figure was generated using Microsoft Word 2007 (Microsoft, Redmond, WA, USA).
Figure 1. Flow diagram demonstrating the patient selection process in this study. The figure was generated using Microsoft Word 2007 (Microsoft, Redmond, WA, USA). 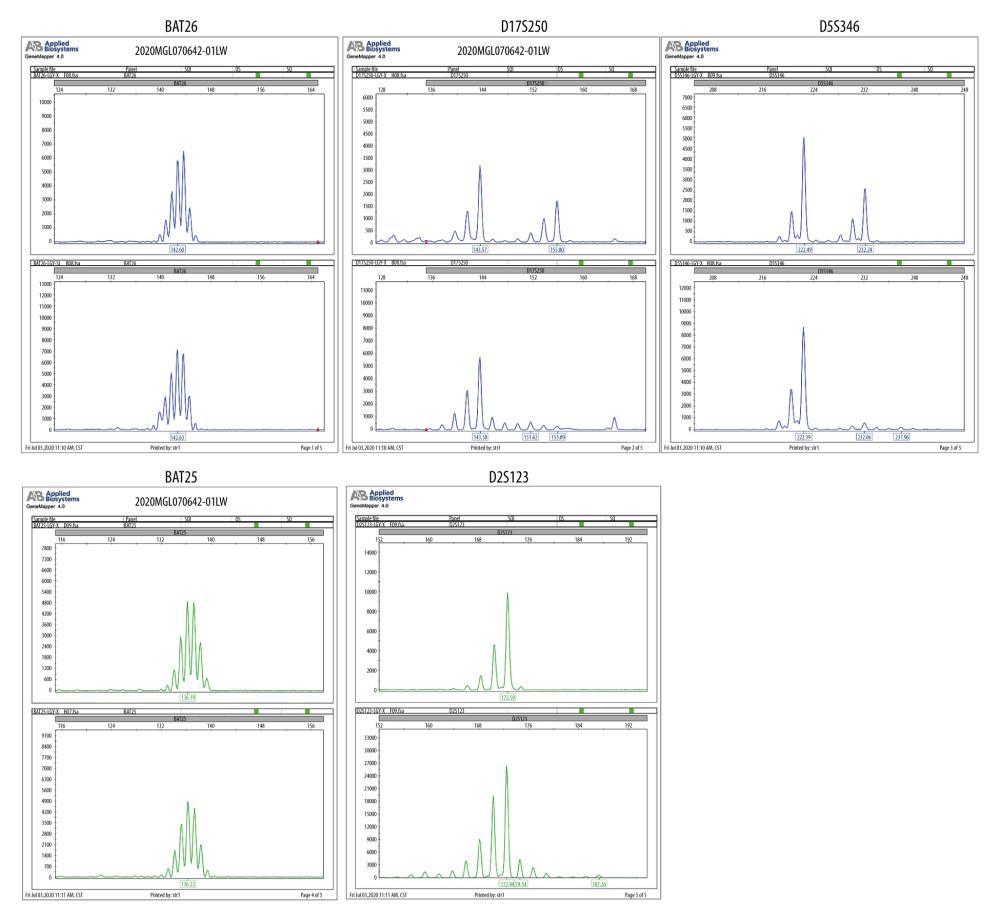 Figure 2. Tissue samples were tested by capillary electrophoresis to confirmation MSI. The figure was edited using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA) from a screen shot from the Applied Biosystems (ABI 3500 DX) in Shanghai Tech Laboratory Co., Ltd. (Shanghai, China).
Figure 2. Tissue samples were tested by capillary electrophoresis to confirmation MSI. The figure was edited using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA) from a screen shot from the Applied Biosystems (ABI 3500 DX) in Shanghai Tech Laboratory Co., Ltd. (Shanghai, China).  Figure 3. (A–D) Patients were diagnosed with advanced endometrial cancer by MRI. The figure was edited using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA) from a screenshot from the Medixant, RadiAnt DICOM Viewer (version 2020.2.3) in our institution.
Figure 3. (A–D) Patients were diagnosed with advanced endometrial cancer by MRI. The figure was edited using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA) from a screenshot from the Medixant, RadiAnt DICOM Viewer (version 2020.2.3) in our institution. 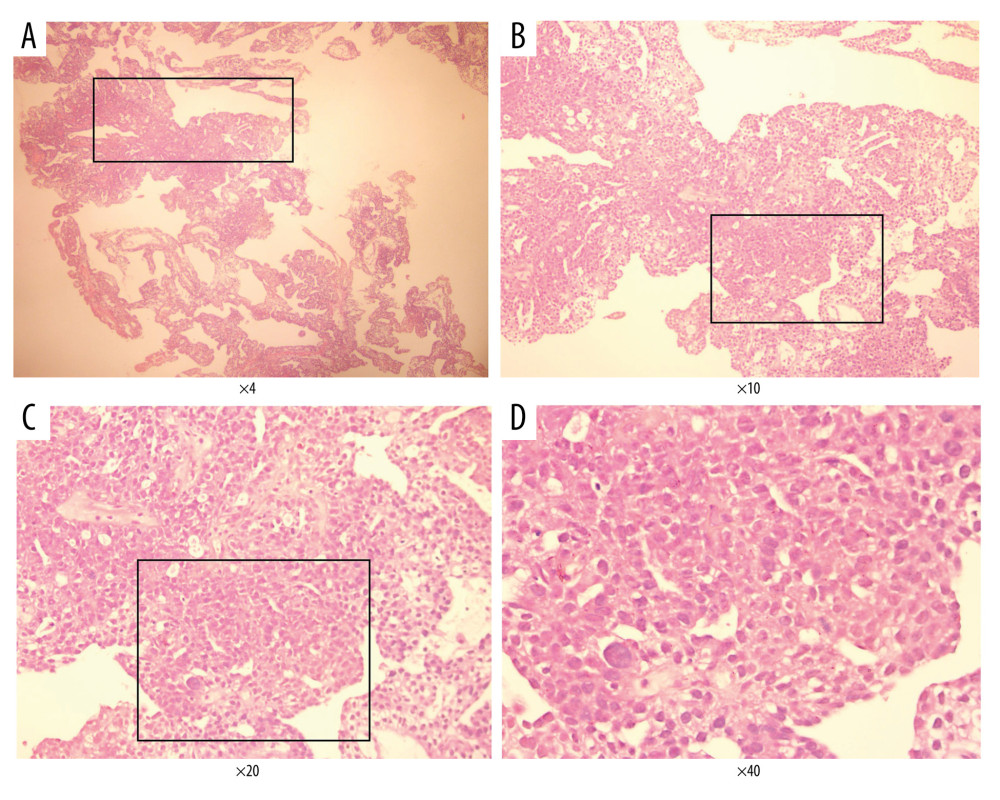 Figure 4. Endometrial cancer tissue samples were confirmed by histological analysis (H&E staining). A–D, represents different magnification (Magnification ×4, ×10, ×20, ×40). The Figure was created by Adobe Illustrator CC 2019, Adobe Systems Incorporated.
Figure 4. Endometrial cancer tissue samples were confirmed by histological analysis (H&E staining). A–D, represents different magnification (Magnification ×4, ×10, ×20, ×40). The Figure was created by Adobe Illustrator CC 2019, Adobe Systems Incorporated. 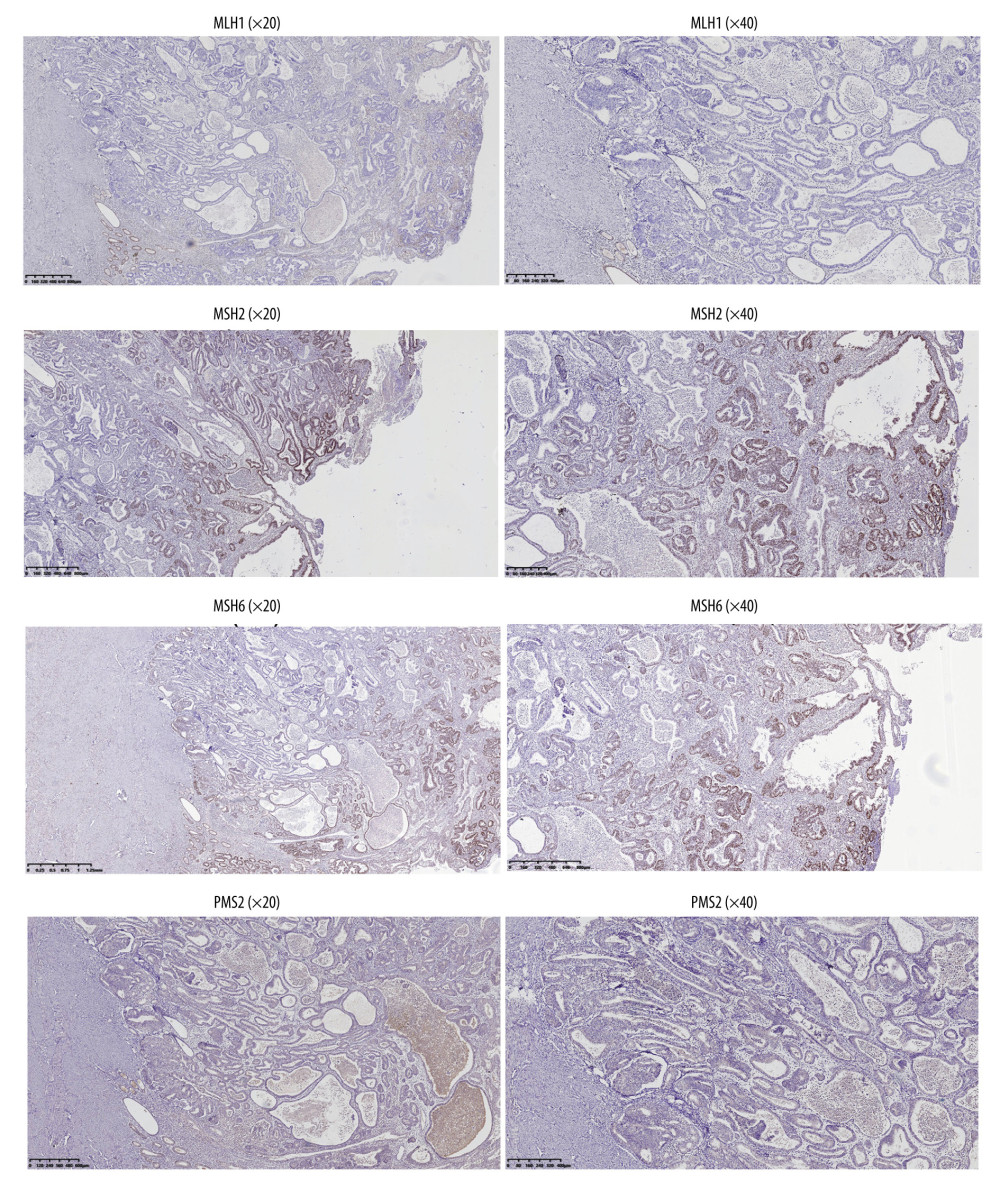 Figure 5. The dMMR proteins in endometrial cancer tissue samples were stained for immunohistochemistry. The figure was created using Adobe Illustrator CC 2019, Adobe Systems Incorporated.
Figure 5. The dMMR proteins in endometrial cancer tissue samples were stained for immunohistochemistry. The figure was created using Adobe Illustrator CC 2019, Adobe Systems Incorporated. 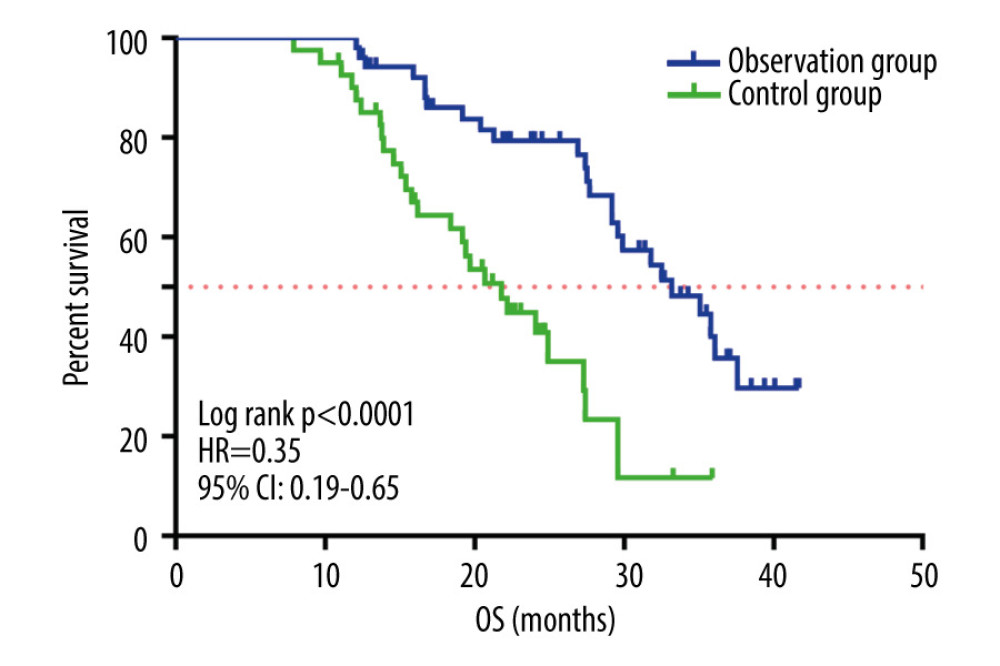 Figure 6. Kaplan-Meier survival curve of patients in the 2 groups. The figure was created using GraphPad Prism version 7.0 software.
Figure 6. Kaplan-Meier survival curve of patients in the 2 groups. The figure was created using GraphPad Prism version 7.0 software. 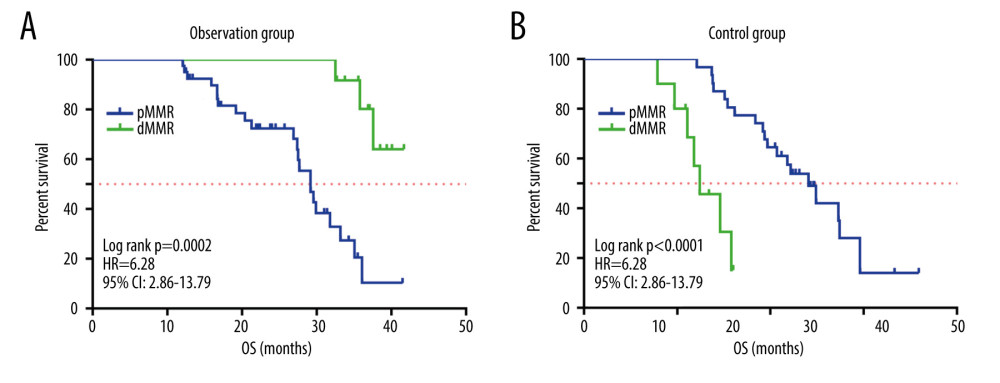 Figure 7. (A, B) Kaplan-Meier survival curves for patients with MMR status in the 2 groups. The figure was created using GraphPad Prism version 7.0 software.
Figure 7. (A, B) Kaplan-Meier survival curves for patients with MMR status in the 2 groups. The figure was created using GraphPad Prism version 7.0 software. References
1. Zhang W, Qiu X, Sun D, systematic analysis of the clinical relevance of cell division cycle associated family in endometrial carcinoma: J Cancer, 2020; 11(19); 5588-600
2. Pecorelli S, Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium: Int J Gynaecol Obstet, 2009; 105(2); 103-4 [Erratum in: Int J Gynaecol Obstet. 2010;108(2):176]
3. Siegel RL, Miller KD, Jemal A, Cancer statistics, 2017: Cancer J Clin, 2017; 67(1); 7-30
4. Kahn RM, Gordhandas S, Maddy BP, Universal endometrial cancer tumor typing: How much has immunohistochemistry, microsatellite instability, and MLH1 methylation improved the diagnosis of Lynch syndrome across the population?: Cancer, 2019; 125(18); 3172-83
5. Broaddus RR, Luthra R, Lu KH, Utility of MLH1 methylation analysis in the clinical evaluation of lynch syndrome in women with endometrial cancer: Current Pharmaceutical Design, 2014; 20(11); 1655-63
6. Amant F, Mirza MR, Koskas M, Cancer of the corpus uteri: Int J Gynecol Obstet, 2018; 143; 37-50
7. Minion LE, Tewari KS, Cervical cancer – State of the science: From angiogenesis blockade to checkpoint inhibition: Gynecol Oncol, 2018; 148(3); 609-21
8. Fader AN, Diaz LA, Armstrong DK, Preliminary results of a phase II study: PD-1 blockade in mismatch repair–deficient, recurrent or persistent endometrial cancer: Gynecol Oncol, 2016; 141; 206-7
9. Ott PA, Bang YJ, Berton-Rigaud D, Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: Results from the KEYNOTE-028 study: J Clin Oncol, 2017; 35(22); 2535-41
10. Le DT, Durham JN, Smith KN, Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade: Science, 2017; 357(6349); 409-13
11. Rose PG, Ali S, Moslemi-Kebria M, Simpkins F, Paclitaxel, carboplatin, and bevacizumab in advanced and recurrent endometrial carcinoma: Int J Gynecol Cancer, 2017; 27(3); 452-58
12. Simpkins F, Drake R, Escobar PF, A phase II trial of paclitaxel, carboplatin, and bevacizumab in advanced and recurrent endometrial carcinoma (EMCA): Gynecol Oncol, 2015; 136(2); 240-45
13. McConechy MK, Talhouk A, Li-Chang HH, Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas: Gynecol Oncol, 2015; 137(2); 306-10
14. Kurman RJ, Carcangiu ML, Herrington CS: WHO classification of tumours of female reproductive organs, 2014; 8-253, Lyon, IARC Press
15. Kandoth C, Schultz N, Cherniack A, Integrated genomic characterization of endometrial carcinoma: Nature, 2013; 497(7447); 67-73
16. Viswanathan AN, Lee H, Berkowitz R, A prospective feasibility study of radiation and concurrent bevacizumab for recurrent endometrial cancer: Gynecol Oncol, 2014; 132(1); 55-60
17. Aghajanian C, Filiaci V, Dizon DS, A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer: Gynecol Oncol, 2018; 150(2); 274-81
18. Lorusso D, Ferrandina G, Colombo N, Carboplatin-paclitaxel compared to Carboplatin-Paclitaxel-Bevacizumab in advanced or recurrent endometrial cancer: MITO END-2 – A randomized phase II trial: Gynecol Oncol, 2019; 155(3); 406-12
19. Tripathi S, Guleria I, Role of PD1/PDL1 pathway, and TH17 and treg cells in maternal tolerance to the fetus: Biomed J, 2015; 38(1); 25-31
20. Ray A, Das DS, Song Y, Targeting PD1-PDL1 immune checkpoint in plasmacytoid dendritic cell interactions with T cells, natural killer cells and multiple myeloma cells: Leukemia, 2015; 29(6); 1441-44
21. O’Donnell JS, Long GV, Scolyer RA, Resistance to PD1/PDL1 checkpoint inhibition: Cancer Treat Rev, 2017; 52; 71-81
22. Laken H, Kehry M, McNeeley P, Identification and characterization of TSR-042, a novel anti-human PD-1 therapeutic antibody: Eur J Cancer, 2016; 1(69); S102
Figures
 Figure 1. Flow diagram demonstrating the patient selection process in this study. The figure was generated using Microsoft Word 2007 (Microsoft, Redmond, WA, USA).
Figure 1. Flow diagram demonstrating the patient selection process in this study. The figure was generated using Microsoft Word 2007 (Microsoft, Redmond, WA, USA). Figure 2. Tissue samples were tested by capillary electrophoresis to confirmation MSI. The figure was edited using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA) from a screen shot from the Applied Biosystems (ABI 3500 DX) in Shanghai Tech Laboratory Co., Ltd. (Shanghai, China).
Figure 2. Tissue samples were tested by capillary electrophoresis to confirmation MSI. The figure was edited using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA) from a screen shot from the Applied Biosystems (ABI 3500 DX) in Shanghai Tech Laboratory Co., Ltd. (Shanghai, China). Figure 3. (A–D) Patients were diagnosed with advanced endometrial cancer by MRI. The figure was edited using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA) from a screenshot from the Medixant, RadiAnt DICOM Viewer (version 2020.2.3) in our institution.
Figure 3. (A–D) Patients were diagnosed with advanced endometrial cancer by MRI. The figure was edited using Microsoft PowerPoint 2010 (Microsoft, Redmond, WA, USA) from a screenshot from the Medixant, RadiAnt DICOM Viewer (version 2020.2.3) in our institution. Figure 4. Endometrial cancer tissue samples were confirmed by histological analysis (H&E staining). A–D, represents different magnification (Magnification ×4, ×10, ×20, ×40). The Figure was created by Adobe Illustrator CC 2019, Adobe Systems Incorporated.
Figure 4. Endometrial cancer tissue samples were confirmed by histological analysis (H&E staining). A–D, represents different magnification (Magnification ×4, ×10, ×20, ×40). The Figure was created by Adobe Illustrator CC 2019, Adobe Systems Incorporated. Figure 5. The dMMR proteins in endometrial cancer tissue samples were stained for immunohistochemistry. The figure was created using Adobe Illustrator CC 2019, Adobe Systems Incorporated.
Figure 5. The dMMR proteins in endometrial cancer tissue samples were stained for immunohistochemistry. The figure was created using Adobe Illustrator CC 2019, Adobe Systems Incorporated. Figure 6. Kaplan-Meier survival curve of patients in the 2 groups. The figure was created using GraphPad Prism version 7.0 software.
Figure 6. Kaplan-Meier survival curve of patients in the 2 groups. The figure was created using GraphPad Prism version 7.0 software. Figure 7. (A, B) Kaplan-Meier survival curves for patients with MMR status in the 2 groups. The figure was created using GraphPad Prism version 7.0 software.
Figure 7. (A, B) Kaplan-Meier survival curves for patients with MMR status in the 2 groups. The figure was created using GraphPad Prism version 7.0 software. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952