24 November 2022: Clinical Research
Multiple Cytokine Analysis of Th1/Th2/Th9/Th17/Th22/Treg Cytokine Pathway for Individual Immune Profile Assessment in Patients with Psoriasis
Anna Michalak-Stoma1ABCDEF*, Joanna Bartosińska2ABCDEF, Dorota Raczkiewicz3BCDEF, Małgorzata Kowal1ABCDE, Joanna Kozak4BCDE, Mariusz Gujski5CDEF, Dorota Krasowska1ACDEF, Grażyna Chodorowska1ADEFDOI: 10.12659/MSM.938277
Med Sci Monit 2022; 28:e938277
Abstract
BACKGROUND: Psoriasis is an autoimmune and autoinflammatory disorder that has a significant impact on patient quality of life. The aim of the study was to assess the immune profiles of patients with psoriasis with multiple cytokine analysis.
MATERIAL AND METHODS: Fifty-two male psoriatic patients and 24 healthy male volunteers were recruited. Granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon gamma (IFN-gamma), interleukin (IL)-1 beta, IL-2, Il-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, IL-17A, IL-18, IL-21, IL-22, IL-23, IL-27, and tumor necrosis factor (TNF)-alpha were measured in patients’ serum with a Th1/Th2/Th9/Th17/Th22/Treg Cytokine 18-Plex Human ProcartaPlex Panel, based on Luminex xMAP technology.
RESULTS: The median fluorescence intensities of serum GM-CSF, IL-2, IL-5, IL-10, IL-13, IL-17A, IL-21, and IL-22 were not intensive enough to calculate the cytokine concentration. We observed elevated levels of IL-6 (P=0.001) and IL-9 (P=0.003) in patients, compared with the control group. The levels of IL-1beta (P=0.008) and IL-27 (P=0.006) were decreased. In patients with psoriatic arthritis, we noticed a decreased level of IL-9 compared with that in patients without arthritis (P=0.034). The levels of IL-12 (P<0.05) and IL-18 (P<0.05) correlated positively with the Psoriasis Area and Severity Index. We found negative correlations of IL-9 (P<0.05), IL-12 (P<0.05), and IL-23 (P<0.05) with the age of psoriatic patients; IL-12 (P<0.05) and IL-23 (P<0.05) with psoriasis duration; and IL-6 (P<0.05) and IL-9 (P<0.05) with the Nail Psoriasis Severity Index.
CONCLUSIONS: Multiple cytokine analysis seems to be an important form of individual immune profile assessment before treatment selection.
Keywords: Autoimmunity, Cytokines, Inflammation Mediators, Psoriasis, Humans, Male, Granulocyte-Macrophage Colony-Stimulating Factor, Interleukin-10, Interleukin-12, Interleukin-13, Interleukin-17, Interleukin-18, Interleukin-2, Interleukin-23, Interleukin-27, Interleukin-5, Interleukin-6, Interleukin-9, Quality of Life, T-Lymphocytes, Regulatory, T-Lymphocytes, Helper-Inducer
Background
Psoriasis is a common disorder that has a significant negative impact on patient quality of life [1]. Therefore, many studies have been conducted to understand its pathogenesis and find effective treatment. Today, psoriasis is considered an autoimmune and autoinflammatory disorder with a genetic and environmental background [2–4]. Excessive activation of a few T helper (Th) cell subpopulations is thought to be central to the inflammatory response in psoriasis [3,5]. Initially, Th1 cells were found as the main source of proinflammatory cytokines, such as interferon (IFN) gamma, tumor necrosis factor (TNF)-alpha, and interleukin (IL)-12. Their differentiation of naïve T cells is stimulated by IL-12 secreted by activated myeloid dendritic cells [5]. Th1 cytokines are found in high levels in lesional skin and in the peripheral blood in patients with psoriasis [4,6–8]. When activated, myeloid dendritic cells also secrete IL-23, which is crucial to the survival and proliferation of Th17 and Th22 cells. The IL-23-mediated Th17 pathway has been declared essential in psoriasis pathogenesis [9]. Th17 cells produce proinflammatory cytokines IL-17A, IL-17F, IL-21, IL-22, IL-25, Il-26, and TNF. These cytokines increase keratinocyte proliferation, upregulate expression of angiogenic mediators and endothelial adhesion molecules, and escalate infiltration of immune cells into lesional skin [2,5]. Th17 cells exacerbate Th1 immune response mainly due to the secretion of IL-17A, which is responsible for the recruitment of neutrophils, activation of innate immune cells, enhancement of B cell functions, and release of proinflammatory cytokines [3,10]. The crucial role of IL-17A in psoriasis was proven by a good clinical response to the therapies downregulating IL-17A in lesional skin [11–14]. Th22 cells represent another subset of human skin-homing memory T cells, which are involved in epidermal immunity and remodeling [3,15]. Th22 cells produce IL-22, IL-13, and IL-26 [3]. IL-6 and TNF-alpha promote the Th22 phenotype [16]. Higher serum levels of IL-22 were observed in patients with psoriasis, compared with healthy controls [17–19]. IL-22 serum levels correlate with Psoriasis Area and Severity Index (PASI) scores [15–17]. Th9 cells are a recently described subset of Th cells that produce IL-9, IL-10, and IL-21 [3]. They develop from naive T cells in the presence of tumor growth factor (TGF)-beta and IL-4 [3]. Studies suggest that Th9 cells have pro-inflammatory and anti-inflammatory functions in autoimmune diseases [20]. Inflammatory and autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematous, and psoriasis, associated with the Th17 pathway, showed high serum levels of IL-9 [20–23].
The recognition of signaling pathways in the pathogenesis of psoriasis is very important for new treatment options. Over the past years, various novel drugs, such as biological or targeted synthetic disease-modifying antirheumatic drugs (DMARDs), were introduced in the treatment of psoriasis and psoriatic arthritis (PsA) [9,24–26]. Biological DMARDs used in psoriasis belong to TNF inhibitors (adalimumab, certolizumab-pegol, etanercept, infliximab, golimumab), IL-12/23 inhibitor (ustekinumab), IL-17 inhibitors (ixekizumab, secukinumab, brodalumab), and IL-23 inhibitors (guselkumab, risankizumab, tildrakizumab) [9,24–26]. TNF inhibitors, IL-12/23 inhibitor, and IL-17 inhibitors are approved for PsA treatment [24,26]. There are also trials in PsA assessing clazakizumab (IL-6 inhibitor), abatacept (CD80/86 inhibitor), aBT-122 (anti-TNF/IL-17a), apremilast (phosphodiesterase-4 inhibitor), and Janus kinase inhibitors (JAKi; tofacitinib, filgotinib) [25,26]. The increasing possibilities of different biological and targeted DMARDs prompt clinicians to consider which drug is best for the individual patient. There are a few head-to-head trials comparing IL-17 inhibitors with TNF inhibitors in PsA, directing IL-17 inhibitor as a better treatment selection for PsA patients with skin involvement [26]. In the treatment recommendations of the European League Against Rheumatism, American College of Rheumatology, and Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, the subtype of PsA, involvement of skin, involvement of nails, and severity of psoriasis were considered; however, the cytokine profile of the patient was not analyzed [27–29]. The European League Against Rheumatism proposed some research questions in their recommendations. Among others, the themes of phenotypes and biomarkers are listed. The American College of Rheumatology expressed the need for more comparative data to inform treatment selection [27,28].
The aim of this study was the assessment of psoriatic patients’ immune profiles with a Th1/Th2/Th9/Th17/Th22/Treg Cytokine 18-Plex Human ProcartaPlex Panel (EPX180-12165-901, Affymetrix, eBioscience/Invitrogen, Thermo Fisher Scientifc, Waltham, MA, USA) based on Luminex xMAP technology [30–32]. We compared the cytokine levels between patients with psoriasis and healthy controls as well as psoriatic patients with PsA vs psoriatic patients without arthritis. We also correlated the cytokine levels with clinical features of psoriatic patients (ie, age, age of psoriasis onset, duration of psoriasis, disease severity measured with PASI, body surface area (BSA), Physician Global Assessment (PGA) scores, and the Nail Psoriasis Severity Index (NAPSI). The PASI scale was first published in 1978 [33] and since then it has been commonly used in clinical practice, research, and clinical trials for the assessment of psoriasis severity and area involvement. The BSA is a common tool for percentage assessment of body area involvement, described also as the „rule of nines” because it is defined as 9% coverage for the head and neck, each arm, anterior and posterior leg, as well as the 4 trunk quadrants and 1% for the genitalia. The BSA can also be estimated by the patient’s handprint: 1 handprint reflects approximately 1% of the BSA [34]
Multiple cytokine analysis of the Th1/Th2/Th9/Th17/Th22/Treg cytokine pathway has not yet been commonly performed. In the PubMed database, we found 3 similar papers [37–39] assessing IFN-gamma, IL-1 receptor antagonist (IL-1RA), IL-2, IL-12(p40), IL-17A, Il-22, and IL-23 [37,38], as well as IL-8, IL-10, and IL-21 [38], in the serum of patients with psoriasis. In the serum of patients with PsA, the following were assessed: IL-1beta, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12(p40), IL-13, IL-15, IL-17, IFN-alpha, IFN-gamma, TNF-alpha, epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (FGF), granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), CCL2 (monocyte chemoattractant protein [MCP-1])/(MCAF), CCL3 (macrophage inflammatory protein [MIP]-1alpha], CCL4 (MIP-1beta), and CCL11 (Eotaxin) [39].
Material and Methods
PATIENTS AND CONTROLS:
The study protocol was approved by the Bioethics Committee of the Lublin Medical University, Poland (approval no. KE-0254/35/2018). The study was conducted in accordance with the guidelines of the Declaration of Helsinki and Good Clinical Practice. Written informed consent was obtained from all participants prior to the study.
Fifty-two male patients with psoriasis with a mean±SD age of 45.9±15 years (range 21–76 years) were enrolled in the study. The diagnosis of psoriasis was made clinically and pathologically (Figure 1A, 1B) [5,40]. The diagnosis of PsA was made by the rheumatologist in accordance with the Classification Criteria for Psoriatic Arthritis (CASPAR) (Figure 1C, 1D) [5,41]. The patients fulfilled the following criteria: minimum 1-year history of psoriasis, no local or systemic treatment for at least 4 weeks prior to entering the study, no significant bacterial or viral infection or suppression of the immune response, and no history of any other significant diseases.
Clinical severity of the disease was evaluated with the PASI, BSA, and PGA scores. Nail involvement was assessed with NAPSI. The patients completed the Dermatology Quality of Life Index form consisting of 10 questions about their perception of the disease impact on different aspects of their health-related quality of life over the last week [42].
As a control group, 24 healthy male volunteers were recruited in this study.
PSORIASIS AREA AND SEVERITY INDEX:
The PASI in all patients was assessed by 1 researcher. Four body regions (head, trunk, upper and lower extremities) were scored individually. The assessment of erythema, infiltration, and desquamation on a scale of 0 (no symptoms) to 4 (very severe symptoms) was performed. The body surface area involvement was assessed over 4 body regions and received a numerical score representing the proportion involved, as follows: 0=no symptoms; 1=1–9%; 2=10–29%; 3=30–49%; 4=50–69%; 5=70–89%; and 6=90–100%. Then, the sum of all 3 severity parameters was calculated, multiplied by the area score, and finally multiplied by the weight of the respective section (0.1 for head, 0.2 for upper extremities, 0.3 for trunk, and 0.4 for lower extremities). The 4 scores were combined into the final PASI [33].
BODY SURFACE AREA:
The BSA in all patients was assessed by 1 researcher. The patient’s full handprint was estimated as 1% of the total body. The percentage of the total body surface area affected by psoriasis was calculated [34].
PHYSICIAN GLOBAL ASSESSMENT:
The PGA in all patients was assessed by 1 researcher. The PGA chosen for this study was a 6-point scale, and the following categories were used: 0=clear, 1=almost clear, 2=mild, 3=moderate, 4=severe, and 5=very severe [35].
NAIL PSORIASIS SEVERITY INDEX:
The NAPSI in all patients was assessed by 1 researcher. Every nail plate was divided into quadrants by imaginary longitudinal and horizontal lines, and the features of nail bed and nail matrix psoriasis were assessed. The features of nail matrix psoriasis were recognized as nail pitting, leukonychia, red spots in the lunula, and crumbling in each quadrant of the nail. The features of nail bed psoriasis were recognized as onycholysis, oil drop dyschromia, splinter hemorrhages, and nail bed hyperkeratosis in each quadrant of the nail. The score was 0 if the findings were not present, 1 if they were present in 1 quadrant of the nail, 2 if present in 2 quadrants of a nail, 3 if present in 3 quadrants of a nail, and 4 if present in 4 quadrants of a nail. Each nail had a matrix score (0–4) and a nail bed score (0–4), and the total nail score was the sum of those 2 individual scores (0–8). The sum of the total score of all involved fingernails was the total NAPSI score for that patient [36].
SERUM SAMPLES:
Venous blood samples (5–10 mL) were collected into vacuum tubes under sterile conditions from the patients and healthy controls. Serum was obtained fresh, spun in a centrifuge, immediately frozen at −70°C, and stored until processed.
MULTIPLE CYTOKINE ANALYSIS:
Serum multiple cytokine analysis was performed using a Th1/Th2/Th9/Th17/Th22/Treg Cytokine 18-Plex Human ProcartaPlex Panel (EPX180-12165-901, Affymetrix, eBioscience/Invitrogen, Thermo Fisher Scientifc, Waltham, MA, USA) based on Luminex xMAP technology, which involves magnetic beads internally color-coded with 2 fluorescent dyes of different intensity. The distinctly colored bead sets are coated with a target-specific capture antibody against the analyte. The assay reagents are free-floating in the solution, and, in this sense, the Luminex xMAP technology is a combination of flow cytometry and sandwich immunoassays [30,31]. In the first step of the experiment, the analytes from the test samples were captured by the beads coated with a target-specific capture antibody. Next, a biotinylated analyte-specific detection antibody was introduced, allowing the detection of specific analytes from the test sample. For analyte quantitation, the beads were incubated with streptavidin-conjugated R-phycoerythrin (SA-PE), the reporter molecule. Finally, the magnetic beads were illuminated to determine the different assays by internal bead color and analyte concentration by measuring the reporter molecule fluorescence [30,32]. This kit comprises all components necessary for the whole assay procedure and was performed only on serum samples that were thawed at room temperature the day of the experiment. The following cytokines were measured: GM-CSF, INF-gamma, IL-1 beta, IL-2, Il-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, IL-17A, IL-18, IL-21, IL-22, IL-23, IL-27, and TNF-alpha. The assay was performed according to the manufacturer’s protocol, as follows. The 18-plex premixed beads were vortexed and sonicated to disperse the aggregates. An amount of 50 μL of prepared beads was added into each well of a 96-well plate, according to the layout. Next, the plate was inserted into the hand-held magnetic plate washer to accumulate the beads on the bottom of each well and to safely remove the liquid. The immobilized beads were washed once using freshly prepared wash buffer, 150 μL per well. The human cytokine lyophilized standard was reconstituted in 250 μL of universal assay buffer, then mixed and incubated on ice for 10 min. The 4-fold serial dilutions were prepared in universal assay buffer (in duplicate). The 7 different standards were prepared with a different concentration range for a specific analyte. Subsequently, 25 μL of universal assay buffer was added into each well of a 96-well plate following the addition of 25 μL of samples or standards into an appropriate well. The plates were incubated with the mixed beads for 2 h at room temperature in the dark while shaking. After incubation, the plate was inserted into the hand-held magnetic plate washer to accumulate the beads captured with specific analyte on the bottom of each well and to remove the liquid. The immobilized complexes were washed twice with 150 μL of wash buffer per well. In the next step, the plate was removed from hand-held magnetic plate washer and 25 μL of detection antibody mixture was added into each well and then incubated for 30 min at room temperature in the dark while shaking. Then, the plate was inserted into the hand-held magnetic plate washer to accumulate the beads on the bottom of each well and to remove the liquid. The beads were washed twice with 150 μL of wash buffer per well. After the washing steps, 50 μL of SA-PE was added into each well. The plate was removed from the hand-held magnetic plate washer and the bead complexes were incubated with SA-PE for an additional 30 min in the same conditions as previously described. After incubation, 2 washes were performed in the same procedure as previously described. Finally, the beads were resuspended in 120 μL per well of reading buffer and shaken for 5 min at room temperature. The individual beads conjugated with analyte were subsequently detected by the Luminex MagPix plate reader (Luminex Technologies, Inc., Austin, TX, USA). The median fluorescence intensity was then compared to the standard curve to calculate the cytokine concentration in pg/mL with xPonent 4.2 software. Five-parameter logistic curve fitting was used for the best curve fit and to derive the analyte concentrations in each sample.
STATISTICAL ANALYSIS:
The data were analyzed using Statistica 13.1 software (Statsoft, Tulsa, OK, USA). All results are given as mean±standard deviation and median with interquartile range (25–75%). We used the Mann-Whitney U test to evaluate the differences between 2 groups (healthy controls vs patients with psoriasis, patients with PsA vs psoriatic patients without arthritis, patients with PsA vs healthy controls, psoriatic patients without arthritis vs healthy controls). Correlation analysis was performed using the Pearson correlation coefficient. Statistical significance was accepted as
Results
The characteristics of the studied groups are presented in Table 1.
We calculated the cytokine concentration based on median fluorescence intensity. Unfortunately, if the analyte concentration was below the lowest standard concentration, we could not obtain the value. Based on instrument settings, to calculate the median fluorescence intensity of each analyte and standards the bead events/counts should be equal or higher than 50. We obtained this value for each of the tested analytes and standards. The information about the bead events/counts for each analyte were generated in the final report of the experiment. Based on those findings, we assumed that the concentrations of GM-CSF, IL-2, IL-5, IL-10, IL-13, IL-17A, IL-21, and IL-22 in tested samples were below the detection range of the used kit and Luminex xMAP technology. The results of comparisons of all measurable cytokine levels between psoriatic patients and the control group are presented in Table 2. We observed significantly elevated levels of IL-6 and IL-9 in psoriatic patients compared with the control group (average level of IL-6: 30.12 vs 17.53 pg/mL,
The results of comparisons of all measurable cytokine levels between psoriatic patients with and without PsA are presented in Table 3. We noticed only a decreased level of IL-9 in patients with PsA compared with the psoriatic patients without arthritis (95.28 vs 145.21 pg/mL on average,
We also compared cytokine levels between patients with PsA and healthy controls, as well as between psoriatic patients without arthritis and healthy controls (Figure 2). We noticed elevated levels of IL-6 and IL-9 in psoriatic patients without arthritis compared with the control group (average level of IL-6: 33.12 vs 17.53 pg/mL,
The age of psoriatic patients correlated negatively with the level of IL-9 (r=−0.36,
The heatmap with dendrograms for psoriatic patients and analyzed cytokines is presented in Figure 6. Visualization is an effect of 2-way hierarchical clustering, where the rows and columns are ordered based on the results of the agglomerative hierarchical clustering, with dendrograms for patients and cytokines shown on the vertical and horizontal axes, respectively. The rows represent patients, and the columns represent cytokines. The darker brown color indicates the higher serum cytokine concentration, whereas the lighter yellow color, the lower cytokine serum concentration. The dendrogram for cytokines is on the top of Figure 6. Cytokines were linked in pairs that are most closely correlated with each other, and therefore provide the same information: IL-18 and IL-4 (r=0.72), IL 9 and IL-6 (r=0.81), and TNF-alpha and IL-1beta (r=0.69). The following cytokines were also correlated, but a little less: IL-18 and IL-4 with IFN-gamma (r=0.71 and 0.53, respectively) and TNF alpha and IL-1beta with IL-27 (r=0.51 and 0.65, respectively). The other pairs were correlated. The dendrogram for psoriatic patients is on the left side of Figure 6. Patients with the most similar concentrations of all analyzed cytokines are paired. The farther away from the heatmap, the less the patients and cytokine similarities are linked.
On the heatmap, the agglomerative hierarchical clustering identified the boundaries of the 5 visual clusters in the network. Patients with numbers 8, 29, 31, 44, 14, 35, 41, 51, 5, 2, 4, and 1 presented high levels of IL-18, IL-4, and IFN-gamma, whereas the levels of IL-9, IL-6, and IL-27 were low. The cluster represents the young patients with severe, long-lasting psoriasis with nail involvement. Patients with numbers 10, 20, 3, 47, and 45 presented high levels of IL-9 and IL-6 and low levels of IL-18, IL-4, IL-27, IL-1beta, and TNF-alpha. None of these patients had PsA. They presented severe psoriasis, but with low NAPSI scores. Patients with numbers 15, 30, 19, 22, and 6 had high levels of TNF-alpha and they presented long-lasting psoriasis, but with lower PASI scores. Patients with numbers 16, 48, 17, 49, 27, 15, 30, 19, 22, 6, 18, 46, 42, 24, 25, 7, 36, 32, and 9 represented the cluster with a high IL-23 level. They presented long-lasting psoriasis, but with lower PASI scores.
Discussion
We performed multiple cytokine analysis of the Th1/Th2/Th9/Th17/Th22/Treg cytokine pathway in psoriatic patients with and without PsA. The following cytokines were measured: GM-CSF, INF-gamma, IL-1 beta, IL-2, Il-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, IL-17A, IL-18, IL-21, IL-22, IL-23, IL-27, and TNF-alpha. However, the median fluorescence intensity of GM-CSF, IL-2, IL-5, IL-10, IL-13, IL-17A, IL-21, and IL-22 was not intensive enough to calculate the cytokine concentrations. We observed significantly elevated levels of IL-6 and IL-9 in psoriatic patients and in psoriatic patients without arthritis, compared with the control group.
In our patients, the results were in accordance with previous studies that demonstrated an increased IL-6 serum level in psoriatic patients compared with healthy controls [4,8,43,44]. The decrease of serum IL-6 level was observed after methotrexate treatment [43], ultraviolet B radiation, and topical steroids [45]. The study by Elango et al showed a positive correlation between IL-6 levels and PASI scores [43]. Furthermore, Lo et al observed that the percentage reduction of PASI scores was correlated with IL-6 serum levels before phototherapy, which can suggest a better response to phototherapy in psoriatic patients with high serum IL-6 [18]. Muramatsu et al examined serum IL-6 levels before and after biological treatment. Infliximab and adalimumab treatment significantly decreased serum IL-6 levels from baseline to the endpoint, while ustekinumab did not affect serum IL-6 levels [46]. We found an elevated level of IL-9 in psoriatic patients; however, patients with PsA presented a significantly lower level of serum IL-9. The study of Cordoso et al indicated that patients with plaque-type psoriasis showed a positive statistically significant difference in serum IL-9 levels compared with healthy controls, and the results suggest that IL-9 might be a good serum biomarker for plaque-type psoriasis [22].
The levels of IL-1beta and IL-27 were decreased in psoriatic patients and in psoriatic patients without arthritis, compared with the control group. Keratinocytes are the main source of IL-1beta in the skin. It is also produced in the adipose tissue, thereby linking inflammation of the skin with obesity [47]. In the present study, the serum levels of IL-1β were decreased in psoriatic patients compared with the control group. No significant difference in serum IL-1β was observed between patients with psoriasis and controls by other authors [47]. However, in the epidermis of psoriatic lesions, IL-1 beta has been shown to be increased, and effective treatment of psoriasis led to a significant decrease in epidermal IL-1 beta expression [48]. The serum level could be lower in our study because of increased production and accumulation of IL-1 beta in patients’ epidermis. Studies comparing the level of IL-1 beta in the epidermis and serum of psoriatic patients should be performed to confirm this hypothesis. In psoriasis, a dual role of IL-27 has been presented. IL-27 can induce certain chemokines in keratinocytes and promote the onset of psoriasis, or it can suppress TNF-alpha-induced cytokines and chemokines and limit the disease [49]. Furthermore, it was shown that IL-27 has an anti-inflammatory action by upregulating IL-18 binding protein (IL-18BP), which is a natural endogenous antagonist of IL-18, in human keratinocytes [50]. IL-27 levels were found to be higher in the serum and lesional skin in psoriatic patients compared with controls and correlated with the severity of the disease [51,52]. Similar results in the serum of plaque-type psoriasis patients from Northeast Brazil were reported by Cardoso et al, and the IL-27 serum level correlated with IL-8 levels [22]. The role of IL-27 was tested in a mouse model of psoriasis and it was shown that IL-27 locally injected in imiquimod-treated psoriasis-like skin lesions enhanced the mRNA levels of Th1 cytokine/chemokines and TNF-alpha without altering those of Th17 cytokine/chemokines. The neutralization of IL-27 during the imiquimod treatment reduced the mRNA levels of Th1 cytokine/chemokines and TNF-alpha and induced clinical and histological improvement [53]. However, Chen et al and Meka et al found that the serum IL-27 level was consistently downregulated in patients with psoriasis compared with the control group [54,55]. In the study of Chen et al, the expressions of IL-27 and IL-27R were also significantly decreased in psoriatic lesions [54]. In our results, the serum level of IL-27 was also decreased, which can suggest an anti-inflammatory role of IL-27.
In the present study, no cytokine levels significantly differed between patients with PsA and healthy controls. We noticed only a decreased level of IL-9 in patients with PsA compared with the psoriatic patients without arthritis. IL-9 is not listed as the main cytokine in the pathogenesis of PsA [56]. Singh et al reported that only approximately one-third of patients with psoriasis have measurable IL-9 levels [21]. These authors found increased IL-9 receptor (IL-9R) expression in psoriatic patients’ skin, particularly at the dermal-epidermal junction, and within the basal layer of the epidermis compared to healthy controls [21]. They also found that there was an increased IL-9R and IL-9 expression in K5.hTGF-b1 mice skin, which presents features typical for psoriasis, such as acanthosis, dermal infiltration by neutrophils, T cells and macrophages, basement membrane degradation, increased angiogenesis, and multiple cytokine abnormalities [21]. The authors observed that in these mice the therapeutic response of PUVA treatment on psoriasiform skin correlated well with the downregulation of IL-9 in the serum [57]. IL-9 signals through the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) system. JAKs are cytoplasmic tyrosine kinases that play an important role in mediating inflammatory responses. Tofacitinib is the JAK inhibitor that modulates immune response by blocking transduction of signals by cytokines, such as IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 [58]. Tofacitinib has demonstrated efficacy in patients with PsA in 2 studies: the phase III Oral Psoriatic Arthritis Trial (OPAL) Broaden–the study of patients with prior inadequate response to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and in the phase III OPAL Beyond–the study in patients with inadequate response to TNF-alpha inhibitors [59,60]. Tofacitinib was approved by the FDA for the treatment of PsA. Intradermal injection of IL-9 in wild mice induced local inflammation and increased expression of IL-17A and STAT3 [21]. Injection with anti-IL-17 antibody stopped the progression of psoriasis in K5.hTGF-b1 transgenic mice and decreased skin IL-9mRNA and serum IL-9 protein levels, suggesting a positive feedback loop between IL-9 and IL-17A [12]. These studies have shown a possible link between the IL-9 and Th17 pathway in psoriasis [12,21]. In addition, IL-9 together with IL-6 and TGF-beta increase the production of IL-17A from cultured and activated CD4+ T cells. These results are consistent with the data on the expression of IL-9R on Th17 cells, suggesting the involvement of Th9 cells in Th17 cell differentiation [3]. IL-9 can also promote the secretion of VEGF from mast cells and keratinocytes and induce angiogenesis [3,21]. IL-9 seems to be only one of the steps in the psoriasis inflammatory cascade, which is why it should not be used as a separate biomarker of psoriasis and PsA.
The age of psoriatic patients correlated negatively with the level of IL-9, IL-12, and IL-23. Psoriasis duration correlated negatively with IL-12 and IL-23. Psoriasis severity measured by the PASI correlated positively with the level of IL-12 and IL-18. IL-23 and IL-12 belong to the IL-12 family and are structurally related; IL-12 consists of p40 and p35 subunits, and IL-23 is built of p40 and p19 subunits [9,61,62]. In psoriasis lesions, mainly the IL-12p40 and IL-23p19 subunits are detected. IL-12 has a direct effect on Th1 differentiation, while IL-23 upregulates IL-17 production and promotes amplification and sustainment of Th17 cells [63]. There are conflicting results of serum IL-12 levels in psoriasis. Some authors reported higher, lower, or not different serum IL-12 levels in psoriasis compared with controls [4,38,61,63–67]. IL-23 serum level has been reported as equal or elevated compared with healthy controls by different authors [64,66–68], Johnston et al showed that a psoriasis-associated polymorphism in the IL12B gene, which encodes the p40 subunit of IL-12 and IL-23, disposed to an increased expression of the IL-12p40 subunit in monocytes and patients with this mutation presented increased serum IL-12 and decreased IL-23 levels [69]. In our present study, a significant positive correlation between IL-12 level and psoriasis severity measured by the PASI (r=0.29,
We found negative a correlation between IL-6 and NAPSI (r=−0.31,
On the heatmap, the pairs IL-18 and IL-4, IL 9 and IL-6, and TNF-alpha and IL-1beta were the pairs most closely correlated with each other. Then, the following cytokines were also correlated, but a little less: IL-18 and IL-4 with IFN-gamma, and TNF alpha and IL-1beta with IL-27. Patients with high levels of IL-18, IL-4, and IFN-gamma and low levels of IL-9 and IL-6 were young and had severe, long-lasting psoriasis with nail involvement. Patients with high levels of IL-9 and IL-6 did not present PsA and had severe psoriasis, but with a low NAPSI score.
Patients with high levels of TNF-alpha presented long-lasting psoriasis, but with lower PASI scores. Patients with high IL-23 level also presented long-lasting psoriasis, but with lower PASI scores.
In previous multiple cytokine analyses, attempts were made to assess the cytokine profile according to the type of psoriasis (guttate vs plaque), clinical phenotype (eruptive inflammatory vs chronic stable psoriasis), or based on swollen joint counts in PsA [37–39]. The serum levels of IL-1RA, IL-2, IL-23, and IFN-gamma were elevated in patients with psoriasis compared with the control group; however, the serum levels of all tested cytokines did not differ significantly between the types of psoriasis, guttate vs plaque [37]. In the Choe et al study, the levels of IL-12, IL-21, IL-22, IL-23, and IL-2 were elevated in patients with psoriasis. They noticed a statistically significant difference between IL-1RA, IL-12, and IL-21 levels in eruptive inflammatory psoriasis compared with the healthy control group and between IL-12 and IL-21 levels in chronic stable psoriasis compared with the healthy control group [38]. A statistically significant difference was observed between IL-1RA and Il-17A levels in eruptive inflammatory vs chronic stable psoriasis [38]. Significant correlations between the serum levels of IFN-gamma, IL-12(p40), and IL-22 and PASI scores were found [37,38]. The levels of IL-8 and IL-17A also showed a linear correlation with PASI scores [38]. However, in the eruptive inflammatory group, there was no evidence of a positive correlation between serum cytokines and PASI scores. In patients with PsA, IFN-alpha, IL-10, and IL-13 levels were significantly increased [39]. IFN-alpha, IL-2, TNF-alpha, IL-15, and IL-12p40 were found to distinguish between PsA patients and healthy individuals [39].
The main limitation of this study was that GM-CSF, IL-2, IL-5, IL-10, IL-13, IL-17A, IL-21, and IL-22 analyte concentrations were below the lowest standard concentration, so we could not obtain median fluorescence intensity values for these cytokines. The results obtained with a Th1/Th2/Th9/Th17/Th22/Treg cytokine panel based on Luminex xMAP technology were not validated with any other method, such as skin biopsy tissue or joint fluid sample mRNA detection using PCR. We performed heatmap analysis of cytokines for the individual patients and we tried to classify the patients following a specific cytokine pattern; however, the cluster groups were not large enough for making relevant conclusions.
Conclusions
Multiple cytokine analysis seems to be an important form of immune profile assessment. To the best of our knowledge, to date, this kind of analysis has not been performed using the Th1/Th2/Th9/Th17/Th22/Treg Cytokine 18-Plex Human ProcartaPlex panel. We observed results for different cytokines at the same time during one analysis, which is probably better than separate assessments of selected cytokines. It can be used to assess the individual immune profile of every patient; for example, before treatment selection, especially with biologics. Based on the heatmap analysis, we think that a larger study group would help differentiate some groups of patients with a similar cytokine profile and help identify the next step in understanding better or worse treatment effects in selected patients.
Figures
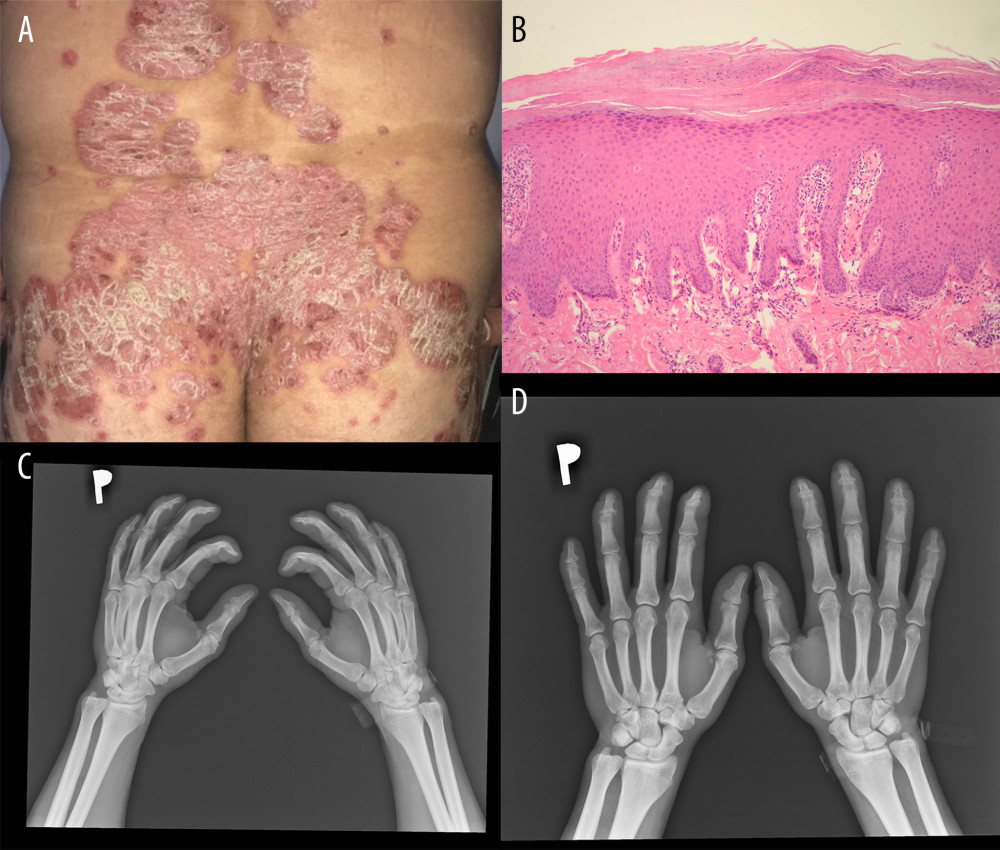 Figure 1. Clinical, pathological, and radiological manifestations of psoriasis. (A) Clinical presentation. (B) Pathological picture of psoriasis. Hematoxylin and eosin, 100× (C, D) Radiological pictures of psoriasis arthritis.
Figure 1. Clinical, pathological, and radiological manifestations of psoriasis. (A) Clinical presentation. (B) Pathological picture of psoriasis. Hematoxylin and eosin, 100× (C, D) Radiological pictures of psoriasis arthritis. 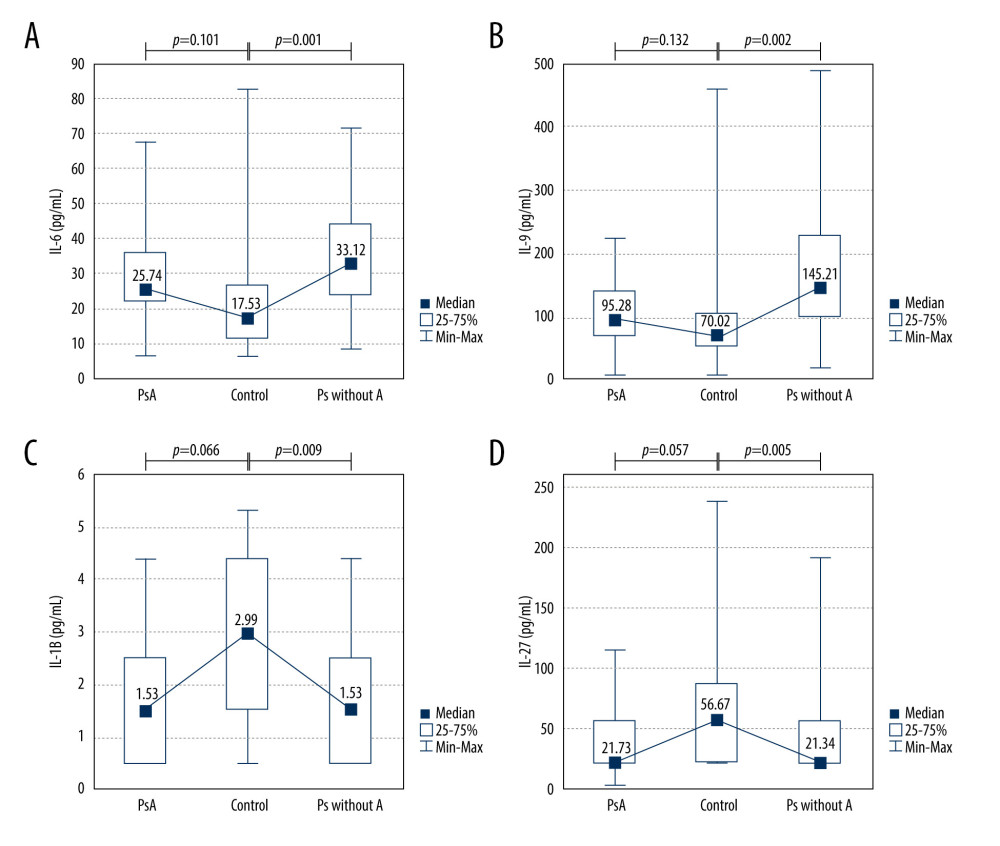 Figure 2. Cytokine levels in the psoriatic patients with (n=14) and without arthritis (n=38) compared with the control group (n=24). (A) IL-6 (average level of IL-6: 33.12 vs 17.53 pg/mL, P=0.001); (B) IL-9 (average level of IL-9: 145.21 vs 70.02 pg/mL, P=0.002); (C) IL-1beta (average level of IL-1beta: 1.53 vs 2.99, P=0.009); (D) IL-27 (average level of IL-27: 21.34 vs 56.67 pg/mL, P=0.005). Ps without A – psoriasis without arthritis; PsA – psoriatic arthritis.
Figure 2. Cytokine levels in the psoriatic patients with (n=14) and without arthritis (n=38) compared with the control group (n=24). (A) IL-6 (average level of IL-6: 33.12 vs 17.53 pg/mL, P=0.001); (B) IL-9 (average level of IL-9: 145.21 vs 70.02 pg/mL, P=0.002); (C) IL-1beta (average level of IL-1beta: 1.53 vs 2.99, P=0.009); (D) IL-27 (average level of IL-27: 21.34 vs 56.67 pg/mL, P=0.005). Ps without A – psoriasis without arthritis; PsA – psoriatic arthritis. 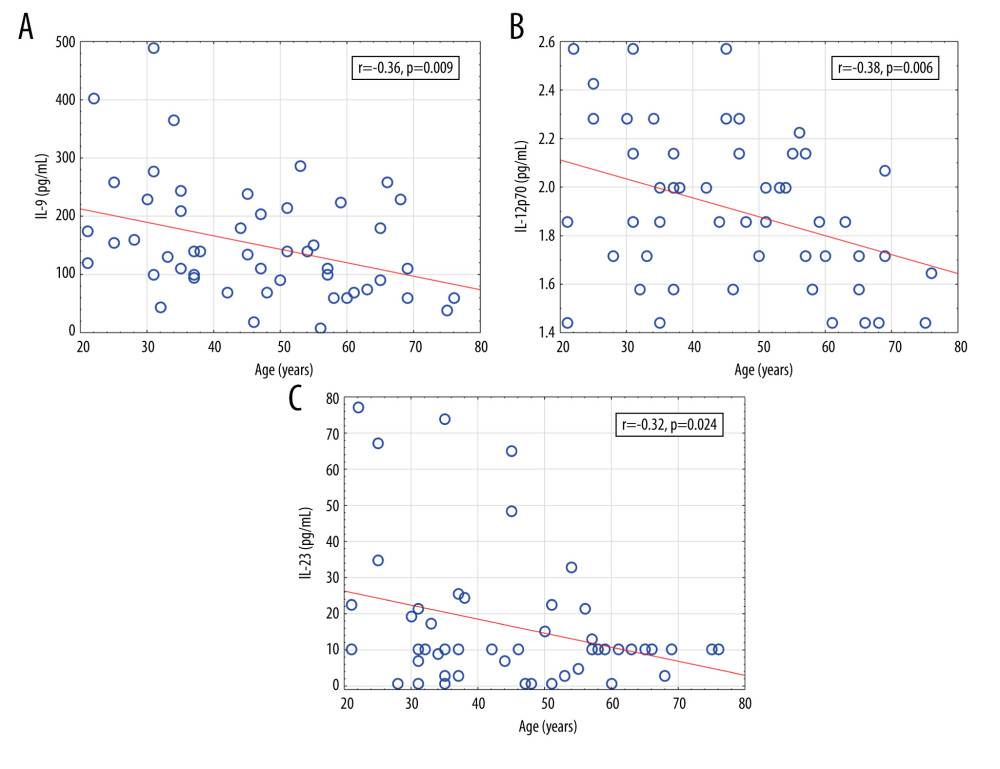 Figure 3. Significant correlations of cytokine levels with age of psoriatic patients (n=52). (A) IL-9 (r=−0.36, P=0.009); (B) IL-12 (r=−0.38, P=0.006); (C) IL-23 (r=−0.32, P=0.024). r – Pearson correlation coefficient.
Figure 3. Significant correlations of cytokine levels with age of psoriatic patients (n=52). (A) IL-9 (r=−0.36, P=0.009); (B) IL-12 (r=−0.38, P=0.006); (C) IL-23 (r=−0.32, P=0.024). r – Pearson correlation coefficient. 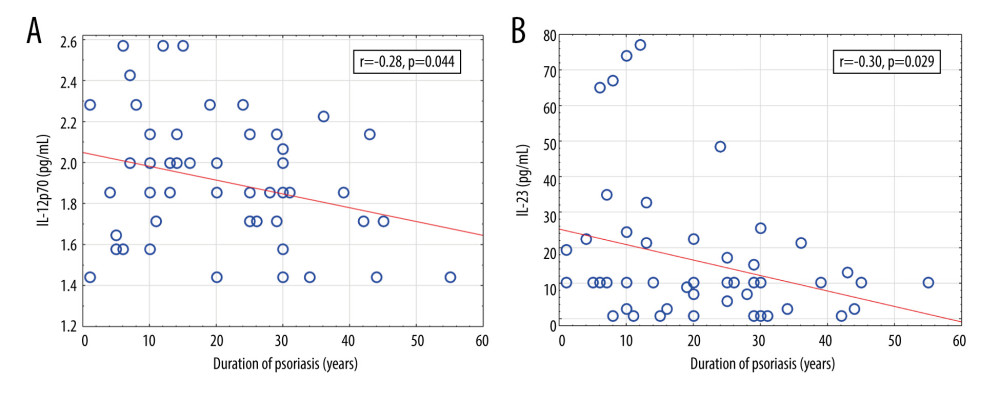 Figure 4. Significant correlations of cytokine levels with psoriasis duration in psoriatic patients (n=52). (A) IL-12 (r=−0.28, P=0.044); (B) IL-23 (r=−0.30, P=0.029). r – Pearson correlation coefficient.
Figure 4. Significant correlations of cytokine levels with psoriasis duration in psoriatic patients (n=52). (A) IL-12 (r=−0.28, P=0.044); (B) IL-23 (r=−0.30, P=0.029). r – Pearson correlation coefficient. 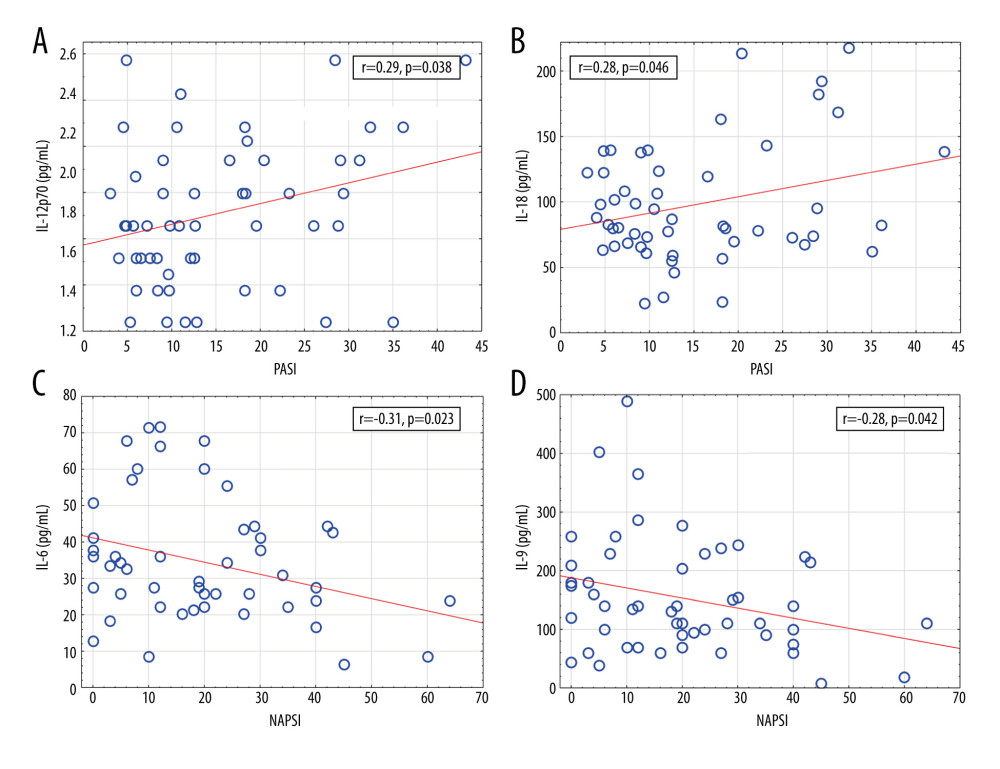 Figure 5. Significant correlations of cytokine levels with Psoriasis Area and Severity Index and Nail Psoriasis Severity Index in psoriatic patients (n=52). (A) IL-12 (r=0.29, P=0.038); (B) IL-18 (r=0.28, P=0.046); (C) IL-6 (r=−0.31, P=0.023); (D) IL-9 (r=−0.28, P=0.042). r – Pearson correlation coefficient.
Figure 5. Significant correlations of cytokine levels with Psoriasis Area and Severity Index and Nail Psoriasis Severity Index in psoriatic patients (n=52). (A) IL-12 (r=0.29, P=0.038); (B) IL-18 (r=0.28, P=0.046); (C) IL-6 (r=−0.31, P=0.023); (D) IL-9 (r=−0.28, P=0.042). r – Pearson correlation coefficient. 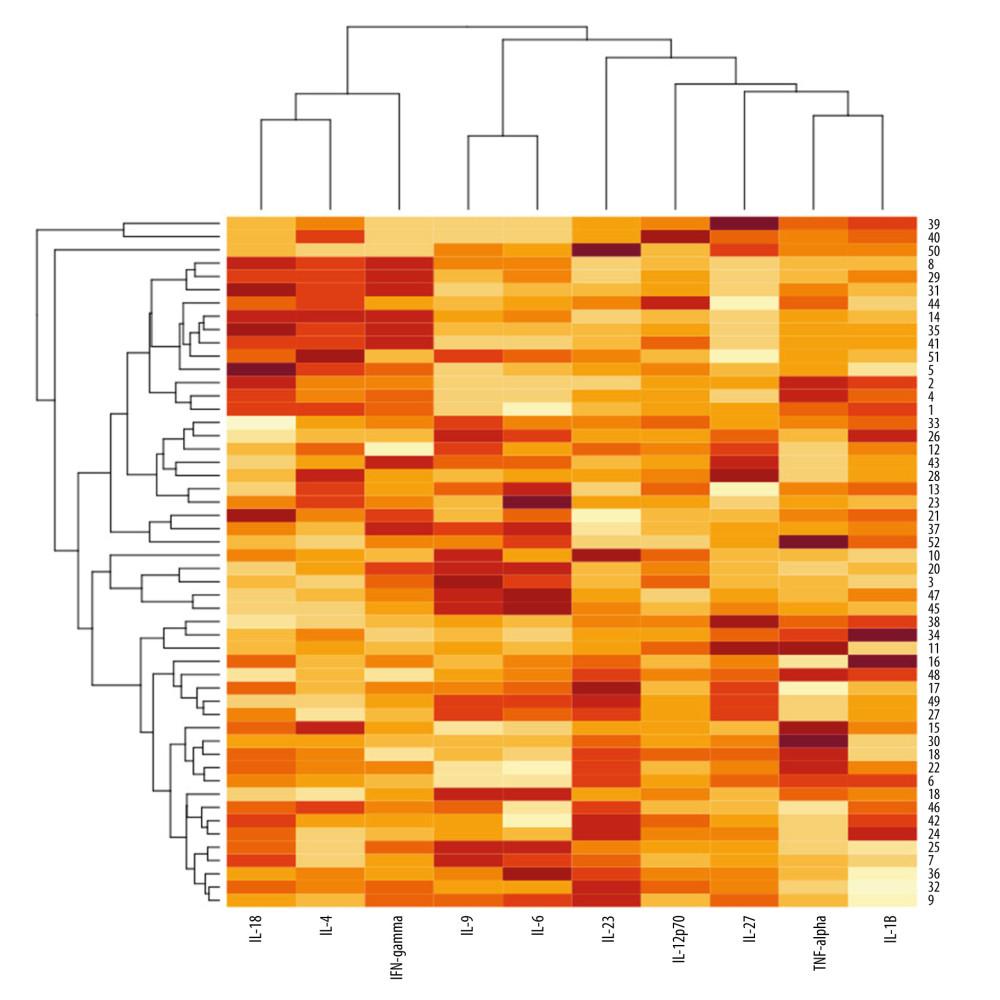 Figure 6. A heatmap with dendrograms for psoriatic patients and analyzed cytokines. Visualization is an effect of 2-way hierarchical clustering, where the rows and columns are ordered based on the results of the agglomerative hierarchical clustering, with dendrograms for patients and cytokines shown on the vertical and horizontal axes, respectively. The rows represent patients, and the columns represent cytokines. The darker brown color represents the higher serum cytokine concentration, whereas the lighter yellow color represents the lower cytokine serum concentration.
Figure 6. A heatmap with dendrograms for psoriatic patients and analyzed cytokines. Visualization is an effect of 2-way hierarchical clustering, where the rows and columns are ordered based on the results of the agglomerative hierarchical clustering, with dendrograms for patients and cytokines shown on the vertical and horizontal axes, respectively. The rows represent patients, and the columns represent cytokines. The darker brown color represents the higher serum cytokine concentration, whereas the lighter yellow color represents the lower cytokine serum concentration. References
1. Bhosle MJ, Kulkarni A, Feldman SR, Balkrishnan R, Quality of life in patients with psoriasis: Health Qual Life Outcomes, 2006; 4; 35
2. Nestle FO, Kaplan DH, Barker J, Mechanisms of disease: Psoriasis: N Engl J Med, 2009; 361; 496-509
3. Karczewski J, Dobrowolska A, Rychlewska-Hańczewska A, Adamski Z, New insights into the role of T cells in pathogenesis of psoriasis and psoriatic arthritis: Autoimmunity, 2016; 49; 435-50
4. Arican O, Aral M, Sasmaz S, Ciragil P, Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity: Mediators Inflamm, 2005; 5; 273-79
5. Armstrong AW, Read C, Pathophysiology, clinical presentation, and treatment of psoriasis: A review: JAMA, 2020; 323; 1945-60
6. Schlaak JF, Buslau M, Jochum W, T cells involved in psoriasis vulgaris belong to the Th1 subset: J Invest Dermatol, 1994; 102; 145-49
7. Khandpur S, Gupta V, Das D, Sharma A, Is there a correlation of serum and tissue T helper-1 and -2 cytokine profiles with psoriasis activity and severity? A cross-sectional study: Indian J Dermatol Venereol Leprol, 2018; 84; 414-18
8. Takahashi H, Tsuji H, Hashimoto Y, Serum cytokines and growth factor levels in Japanese patients with psoriasis: Clin Exp Dermatol, 2010; 35; 645-49
9. Girolomoni G, Strohal R, Puig L, The role of IL-23 and the IL-23/TH 17 immune axis in the pathogenesis and treatment of psoriasis: J Eur Acad Dermatol Venereol, 2017; 31; 1616-26
10. Raphael I, Nalawade S, Eagar TN, Forsthuber TG, T cell subsets and their signature cytokines in autoimmune and inflammatory diseases: Cytokine, 2015; 74; 5-17
11. Zaba LC, Suárez-Fariñas M, Fuentes-Duculan J, Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes: J Allergy Clin Immunol, 2009; 124; 1022-30
12. Singh TP, Schön MP, Wallbrecht K, 8-methoxypsoralen plus ultraviolet A therapy acts via inhibition of the IL-23/Th17 axis and induction of Foxp3+ regulatory T cells involving CTLA4 signaling in a psoriasis-like skin disorder: J Immunol, 2010; 184; 7257-67
13. Langley RG, Elewski BE, Lebwohl M, Secukinumab in plaque psoriasis – results of two phase 3 trials: N Engl J Med, 2014; 371; 326-38
14. McInnes IB, Sieper J, Braun J, Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: A 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial: Ann Rheum Dis, 2014; 73; 349-56
15. Eyerich S, Eyerich K, Pennino D, Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling: J Clin Invest, 2009; 119; 3573-85
16. Duhen T, Geiger R, Jarrossay D, Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells: Nat Immunol, 2009; 10; 857-63
17. Caproni M, Antiga E, Melani L, Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: A randomized-controlled trial: J Clin Immunol, 2009; 29; 210-14
18. Lo YH, Torii K, Saito C, Serum IL-22 correlates with psoriatic severity and serum IL-6 correlates with susceptibility to phototherapy: J Dermatol Sci, 2010; 58; 225-27
19. Meephansan J, Ruchusatsawat K, Sindhupak W, Effect of methotrexate on serum levels of IL-22 in patients with psoriasis: Eur J Dermatol, 2011; 21; 501-4
20. Deng Y, Wang Z, Chang C, Th9 cells and IL-9 in autoimmune disorders: Pathogenesis and therapeutic potentials: Hum Immunol, 2017; 78; 120-28
21. Singh TP, Schön MP, Wallbrecht K, Involvement of IL-9 in Th17-associated inflammation and angiogenesis of psoriasis: PLoS One, 2013; 8; e51752
22. Cardoso PR, Lima EV, Lima MM, Clinical and cytokine profile evaluation in Northeast Brazilian psoriasis plaque-type patients: Eur Cytokine Netw, 2016; 27; 1-5
23. Dantas AT, Marques CDL, Rocha LF, Increased serum Interleukin9 levels in rheumatoid arthritis and systemic lupus erythematosus: Pathogenic role or just an epiphenomenon?: Dis Markers, 2015; 2015; 519638
24. Nast A, Smith C, Spuls P: J Eur Acad Dermatol Venerol, 2020; 34; 2461-98
25. Kerschbaumer A, Smolen JS, Dougados M, Pharmacological treatment of psoriatic arthritis: A systematic literature research for the 2019 update of the EULAR recommendations for the management of psoriatic arthritis: Ann Rheum Dis, 2020; 79; 778-86
26. Vivekanantham A, McGagh D, Coates LC, Current treatments and recommendations for psoriatic arthritis: Best Pract Res Clin Rheumatol, 2021; 35; 101680
27. Gossec L, Baraliakos X, Kerschbaumer A, EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update: Ann Rheum Dis, 2020; 79; 700-12
28. Singh JA, Guyatt G, Ogdie A, Special article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis: Arthritis Care Res, 2019; 71; 2-29
29. Coates LC, Kavanaugh A, Mease PJ, Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis: Arthritis Rheumatol, 2016; 68; 1060-71
30. Adamcova M, Šimko F, Multiplex biomarker approach to cardiovascular diseases: Acta Pharmacol, 2018; 39; 1068-72
31.
32. Butterfield LH, Potter DM, Kirkwood JM, Multiplex serum biomarker assessments: Technical and biostatistical issues: J Transl Med, 2011; 9; 173
33. Fredriksson T, Pettersson U, Oral treatment of pustulosis palmo-plantaris with a new retinoid, Ro 10-9359: Dermatologica, 1979; 158; 60-64
34. Ramsay B, Lawrence CM, Measurement of involved surface area in patients with psoriasis: Br J Dermatol, 1991; 124; 565-70
35. Robinson A, Kardos M, Kimball AB, Physician global assessment (PGA) and psoriasis area and severity index (PASI): Why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis: J Am Acad Dermatol, 2012; 66; 369-75
36. Rich P, Scher RK, Nail Psoriasis Severity Index: A useful tool for evaluation of nail psoriasis: J Am Acad Dermatol, 2003; 49; 206-12
37. Hwang YJ, Jung HJ, Kim MJ, Serum levels of LL-37 and inflammatory cytokines in plaque and guttate psoriasis: Mediators Inflamm, 2014; 2014; 268257
38. Choe YB, Hwang YJ, Hahn HJ, A comparison of serum inflammatory cytokines according to phenotype in patients with psoriasis: Br J Dermatol, 2012; 167; 762-67
39. Szodoray P, Alex P, Chappell-Woodward CM, Circulating cytokines in Norwegian patients with psoriatic arthritis determined by a multiplex cytokine array system: Rheumatology (Oxford), 2007; 46; 417-25
40. Reich A, Adamski Z, Chodorowska G, Psoriasis. Diagnostic and therapeutic recommendations of the Polish Dermatological Society. Part 1: Dermatology Review, 2018; 105; 225-43
41. Taylor W, Gladman D, Helliwell PCASPAR Study Group, Classification criteria for psoriatic arthritis: Development of new criteria from a large international study: Arthritis Rheum, 2006; 54; 2665-73
42. Finlay AY, Khan GK, Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use: Clin Exp Dermatol, 1994; 19; 20-16
43. Elango T, Dayalan H, Subramanian S, Serum interleukin 6 levels in response to methotrexate treatment in psoriatic patients: Clin Chim Acta, 2012; 413; 1652-56
44. Szepietowski JC, Bielicka E, Nockowski P, Increased interleukin-7 levels in the sera of psoriatic patients: Lack of correlations with interleukin-6 levels and disease intensity: Clin Exp Dermatol, 2000; 25; 643-47
45. Bonifati C, Solmone M, Trento E, Serum interleukin-6 levels as an early marker of therapeutic response to UVB radiation and topical steroids in psoriatic patients: Int J Clin Lab Res, 1994; 24; 122-23
46. Muramatsu S, Kubo R, Nishida E, Morita A, Serum interleukin-6 levels in response to biologic treatment in patients with psoriasis: Mod Rheumatol, 2017; 27; 137-41
47. Dowlatshahi EA, van der Voort EAM, Arends LR, Nijsten T, Markers of systemic inflammation in psoriasis: A systematic review and meta-analysis: Br J Dermatol, 2013; 169; 266-82
48. Tsuji G, Hashimoto-Hachiya A, Yen VH, Metformin inhibits IL-1β secretion via impairment of NLRP3 inflammasome in keratinocytes: Implications for preventing the development of psoriasis: Cell Death Discov, 2020; 6; 11
49. Shibata S, Tada Y, Kanda N, Possible roles of IL-27 in the pathogenesis of psoriasis: J Invest Dermatol, 2010; 130; 1034-39
50. Wittmann M, Doble R, Bachmann M, IL-27 Regulates IL-18 binding protein in skin resident cells: PLoS One, 2012; 7; e38751
51. Owaki T, Asakawa M, Morishima N, A role for IL-27 in early regulation of Th1 differentiation: J Immunol, 2005; 175; 2191-200
52. Tojo G, Fujimura T, Kambayashi Y, Aiba S, Systemic lupus erythematosus accompanied by psoriasis induces IL-27-producing cells in both affected areas of the skin: Case Rep Dermatol, 2012; 4; 181-85
53. Shibata S, Tada Y, Asano Y, IL-27 activates Th1-mediated responses in imiquimod-induced psoriasis-like skin lesions: J Invest Dermatol, 2013; 133; 479-88
54. Chen W, Gong Y, Zhang X, Decreased expression of IL-27 in moderate-to-severe psoriasis and its anti-inflammation role in imiquimod-induced psoriasis-like mouse model: J Dermatol Sci, 2017; 85; 115-23
55. Meka RR, Venkatesha SH, Dudics S, IL-27-induced modulation of autoimmunity and its therapeutic potential: Autoimmun Rev, 2015; 14; 1131-41
56. Belasco J, Wei N, Psoriatic arthritis: What is happening at the joint?: Rheumatol Ther, 2019; 6; 305-15
57. Singh TP, Schön MP, Wallbrecht K, 8-methoxypsoralen plus ultraviolet A therapy acts via inhibition of the IL-23/Th17 axis and induction of Foxp3+ regulatory T cells involving CTLA4 signaling in a psoriasis-like skin disorder: J Immunol, 2010; 184; 7257-67
58. Merola JF, Espinoza LR, Fleischmann R, Distinguishing rheumatoid arthritis from psoriatic arthritis: RMD Open, 2018; 4; e000656
59. Mease P, Hall S, FitzGerald O, Tofacitinib or adalimumab versus placebo for psoriatic arthritis: N Engl J Med, 2017; 377; 1537-50
60. Gladman D, Rigby W, Azevedo VF, Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors: N Engl J Med, 2017; 377; 1525-36
61. Pietrzak AT, Zalewska A, Chodorowska G, Cytokines and anticytokines in psoriasis: Clin Chim Acta, 2008; 394; 7-21
62. Duvallet E, Semerano L, Assier E, Interleukin-23: A key cytokine in inflammatory diseases: Ann Med, 2011; 43; 503-11
63. Baliwag J, Barnes DH, Johnston A, Cytokines in psoriasis: Cytokine, 2015; 73; 342-50
64. Kyriakou A, Patsatsi A, Vyzantiadis TA, Sotiriadis D, Serum levels of TNF-α, IL-12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: A cross-sectional study: ScientificWorldJournal, 2014; 2014; 508178
65. Jacob SE, Nassiri M, Kerdel FA, Vincek V, Simultaneous measurement of multiple Th1 and Th2 serum cytokines in psoriasis and correlation with disease severity: Mediators Inflamm, 2003; 12; 309-13
66. Brito-Luna MJ, Villanueva-Quintero DG, Sandoval-Talamantes AK, Correlation of IL-12, IL-22, and IL-23 in patients with psoriasis and metabolic syndrome. Preliminary report: Cytokine, 2016; 85; 130-36
67. Michalak-Stoma A, Bartosińska J, Kowal M, Serum levels of selected Th17 and Th22 cytokines in psoriatic patients: Dis Markers, 2013; 35; 625-31
68. Bajaj S, Gautam RK, Khurana A, Effect of narrow band ultraviolet B phototherapy on T helper 17 cell specific cytokines (interleukins-17, 22 and 23) in psoriasis vulgaris: J Dermatolog Treat, 2017; 28; 14-17
69. Johnston A, Xing X, Swindell WR, Susceptibility-associated genetic variation at IL12B enhances Th1 polarization in psoriasis: Hum Mol Genet, 2013; 22; 1807-15
70. Benezeder T, Wolf P, Resolution of plaque-type psoriasis: What is left behind (and reinitiates the disease): Semin Immunopathol, 2019; 41; 633-44
71. Lee J, Cho D, Park H, IL-18 and cutaneous inflammatory diseases: Int J Mol Sci, 2015; 16; 29357-69
72. Gangemi S, Merendino R, Guarneri F, Serum levels of interleukin-18 and s-ICAM-1 in patients affected by psoriasis: Preliminary considerations: J Eur Acad Dermatol Venerol, 2003; 17; 42-46
73. Ohta Y, Hamada Y, Katsuoka K, Expression of IL-18 in psoriasis: Arch Dermatol Res, 2001; 293; 334-42
74. Flisiak I, Klepacki A, Chodynicka B, Plasma and scales levels of interleukin 18 in comparison with other possible clinical and laboratory biomarkers of psoriasis activity: Biomarkers, 2006; 11; 194-200
75. Pietrzak A, Lecewicz-Torun B, Chodorowska G, Rolinski J, Interleukin-18 levels in the plasma of psoriatic patients correlate with the extent of skin lesions and the PASI score: Acta Derm Venereol, 2003; 83; 262-65
76. Belasco J, Louie JS, Gulati N, Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis: Arthritis Rheumatol, 2015; 67; 934-44
77. Celis R, Planell N, Fernández-Sueiro JL, Synovial cytokine expression in psoriatic arthritis and associations with lymphoid neogenesis and clinical features: Arthritis Res Ther, 2012; 14; R93
78. Thompson C, Davies R, Choy E, Anti cytokine therapy in chronic inflammatory arthritis: Cytokine, 2016; 86; 92-99
79. Mease PJ, Gottlieb AB, Berman A, The efficacy and safety of clazakizumab, an anti-Interleukin-6 monoclonal antibody, in a phase IIb study of adults with active psoriatic arthritis: Arthritis Rheumatol, 2016; 68; 2163-73
80. Costa L, Caso F, Cantarini L, Efficacy of tocilizumab in a patient with refractory psoriatic arthritis: Clin Rheumatol, 2014; 33; 1355-57
81. Hughes M, Chinoy H, Successful use of tocilizumab in a patient with psoriatic arthritis: Rheumatology, 2013; 52; 1728-29
82. Ogata A, Kumanogoh A, Tanaka T, Pathological role of Interleukin-6 in psoriatic arthritis: Arthritis, 2012; 2012; 273618
Figures
 Figure 1. Clinical, pathological, and radiological manifestations of psoriasis. (A) Clinical presentation. (B) Pathological picture of psoriasis. Hematoxylin and eosin, 100× (C, D) Radiological pictures of psoriasis arthritis.
Figure 1. Clinical, pathological, and radiological manifestations of psoriasis. (A) Clinical presentation. (B) Pathological picture of psoriasis. Hematoxylin and eosin, 100× (C, D) Radiological pictures of psoriasis arthritis. Figure 2. Cytokine levels in the psoriatic patients with (n=14) and without arthritis (n=38) compared with the control group (n=24). (A) IL-6 (average level of IL-6: 33.12 vs 17.53 pg/mL, P=0.001); (B) IL-9 (average level of IL-9: 145.21 vs 70.02 pg/mL, P=0.002); (C) IL-1beta (average level of IL-1beta: 1.53 vs 2.99, P=0.009); (D) IL-27 (average level of IL-27: 21.34 vs 56.67 pg/mL, P=0.005). Ps without A – psoriasis without arthritis; PsA – psoriatic arthritis.
Figure 2. Cytokine levels in the psoriatic patients with (n=14) and without arthritis (n=38) compared with the control group (n=24). (A) IL-6 (average level of IL-6: 33.12 vs 17.53 pg/mL, P=0.001); (B) IL-9 (average level of IL-9: 145.21 vs 70.02 pg/mL, P=0.002); (C) IL-1beta (average level of IL-1beta: 1.53 vs 2.99, P=0.009); (D) IL-27 (average level of IL-27: 21.34 vs 56.67 pg/mL, P=0.005). Ps without A – psoriasis without arthritis; PsA – psoriatic arthritis. Figure 3. Significant correlations of cytokine levels with age of psoriatic patients (n=52). (A) IL-9 (r=−0.36, P=0.009); (B) IL-12 (r=−0.38, P=0.006); (C) IL-23 (r=−0.32, P=0.024). r – Pearson correlation coefficient.
Figure 3. Significant correlations of cytokine levels with age of psoriatic patients (n=52). (A) IL-9 (r=−0.36, P=0.009); (B) IL-12 (r=−0.38, P=0.006); (C) IL-23 (r=−0.32, P=0.024). r – Pearson correlation coefficient. Figure 4. Significant correlations of cytokine levels with psoriasis duration in psoriatic patients (n=52). (A) IL-12 (r=−0.28, P=0.044); (B) IL-23 (r=−0.30, P=0.029). r – Pearson correlation coefficient.
Figure 4. Significant correlations of cytokine levels with psoriasis duration in psoriatic patients (n=52). (A) IL-12 (r=−0.28, P=0.044); (B) IL-23 (r=−0.30, P=0.029). r – Pearson correlation coefficient. Figure 5. Significant correlations of cytokine levels with Psoriasis Area and Severity Index and Nail Psoriasis Severity Index in psoriatic patients (n=52). (A) IL-12 (r=0.29, P=0.038); (B) IL-18 (r=0.28, P=0.046); (C) IL-6 (r=−0.31, P=0.023); (D) IL-9 (r=−0.28, P=0.042). r – Pearson correlation coefficient.
Figure 5. Significant correlations of cytokine levels with Psoriasis Area and Severity Index and Nail Psoriasis Severity Index in psoriatic patients (n=52). (A) IL-12 (r=0.29, P=0.038); (B) IL-18 (r=0.28, P=0.046); (C) IL-6 (r=−0.31, P=0.023); (D) IL-9 (r=−0.28, P=0.042). r – Pearson correlation coefficient. Figure 6. A heatmap with dendrograms for psoriatic patients and analyzed cytokines. Visualization is an effect of 2-way hierarchical clustering, where the rows and columns are ordered based on the results of the agglomerative hierarchical clustering, with dendrograms for patients and cytokines shown on the vertical and horizontal axes, respectively. The rows represent patients, and the columns represent cytokines. The darker brown color represents the higher serum cytokine concentration, whereas the lighter yellow color represents the lower cytokine serum concentration.
Figure 6. A heatmap with dendrograms for psoriatic patients and analyzed cytokines. Visualization is an effect of 2-way hierarchical clustering, where the rows and columns are ordered based on the results of the agglomerative hierarchical clustering, with dendrograms for patients and cytokines shown on the vertical and horizontal axes, respectively. The rows represent patients, and the columns represent cytokines. The darker brown color represents the higher serum cytokine concentration, whereas the lighter yellow color represents the lower cytokine serum concentration. Tables
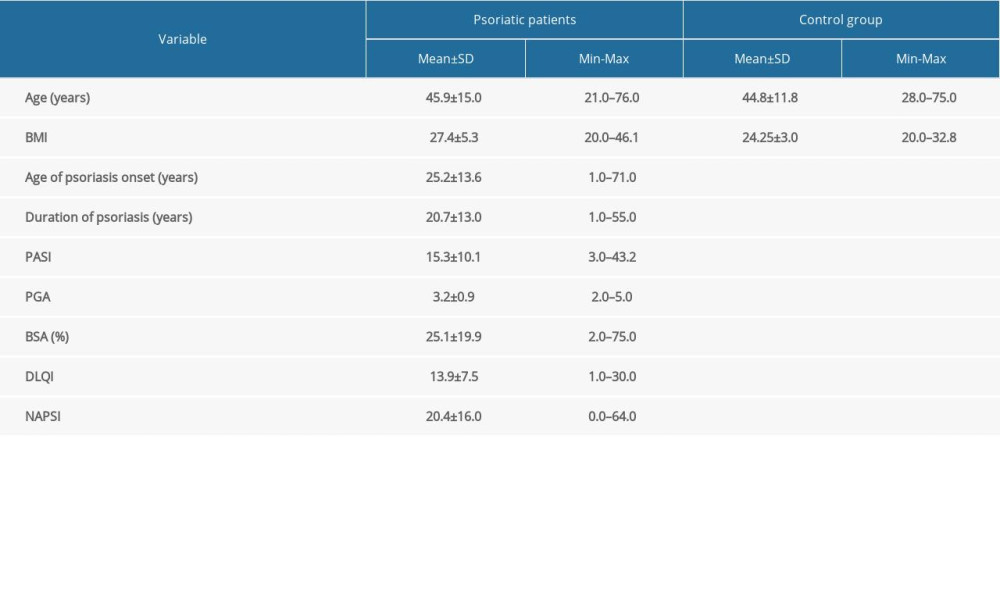 Table 1. The characteristic of the study groups.
Table 1. The characteristic of the study groups.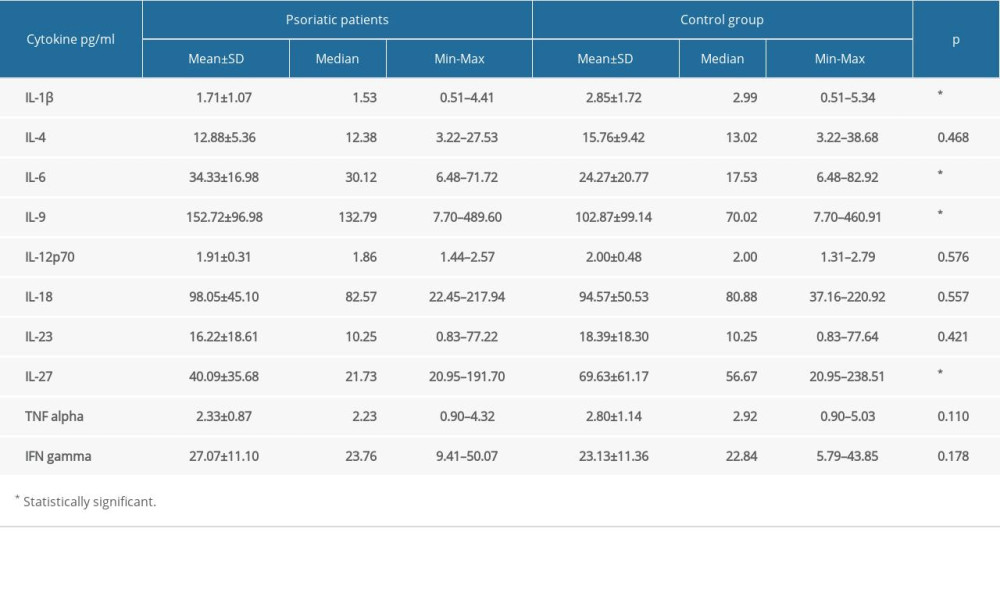 Table 2. Cytokine levels in psoriatic patients and the control group.
Table 2. Cytokine levels in psoriatic patients and the control group.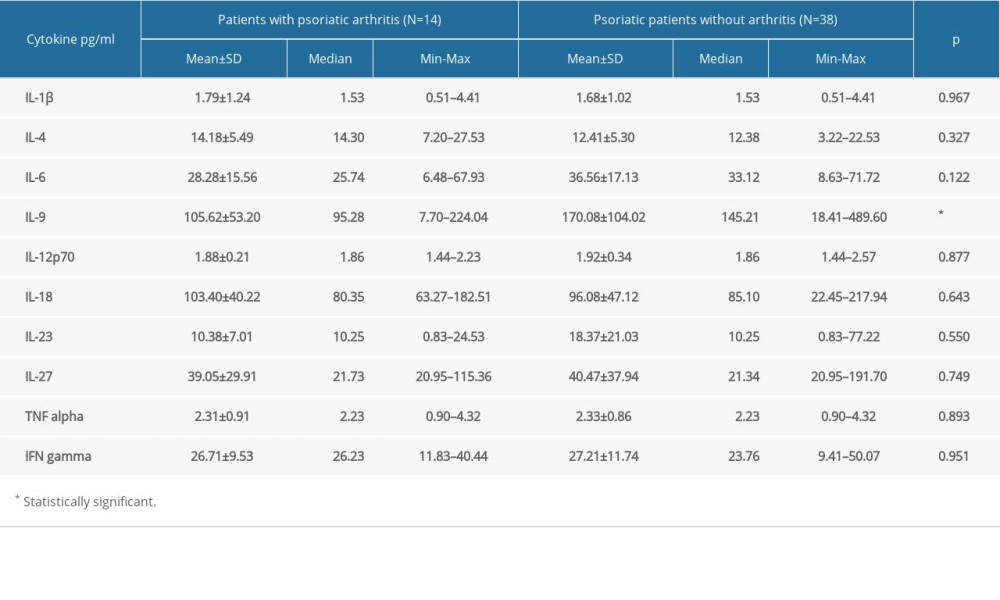 Table 3. Cytokine levels in patients with psoriatic arthritis and psoriatic patients without arthritis.
Table 3. Cytokine levels in patients with psoriatic arthritis and psoriatic patients without arthritis. Table 1. The characteristic of the study groups.
Table 1. The characteristic of the study groups. Table 2. Cytokine levels in psoriatic patients and the control group.
Table 2. Cytokine levels in psoriatic patients and the control group. Table 3. Cytokine levels in patients with psoriatic arthritis and psoriatic patients without arthritis.
Table 3. Cytokine levels in patients with psoriatic arthritis and psoriatic patients without arthritis. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








