04 August 2022: Clinical Research
Hair Sample Analysis of Residents from Olsztyn, Northeastern Poland, to Evaluate Levels of Bisphenol S and Bisphenol A: A Pilot Study
Sławomir Gonkowski1ACDEF, Manolis Tzatzarakis2BCE, Eleni Dermitzaki2BCE, Krystyna Makowska3CE, Joanna Wojtkiewicz4ABEG*DOI: 10.12659/MSM.936738
Med Sci Monit 2022; 28:e936738
Abstract
BACKGROUND: Bisphenol A (BPA) and its analogue bisphenol S (BPS), widely utilized in numerous fields of industry, may seep into the environment and into human organisms. Hitherto, BPA was regarded as the bisphenol to which people were exposed to the greatest extent. As endocrine disruptors, bisphenols have negative effects on human health. Therefore, defining the levels of human exposure to these compounds is a key issue in toxicology. Hair analysis has been increasingly used for biomonitoring of bisphenols in humans, but information about the coexistence of BPA and BPS in human hair is extremely scarce. The present study aimed to analyze hair samples from 25 individuals from Olsztyn, northeastern Poland, to evaluate the levels of these 2 industrial pollutants.
MATERIAL AND METHODS: The method used in the research was liquid chromatography with a mass spectrometry technique.
RESULTS: BPA was found in 72% of samples analyzed and its concentration levels fluctuated from 3.6 to 52.9 ng/g (median 17.7 ng/g). The BPS concentration levels were higher – from 13.4 to 1054.9 ng/g (median 98.7 ng/g). We also found that gender, age, and the presence of artificial hair color (hair dye) did not affect the BPA and BPS levels in the hair.
CONCLUSIONS: This study has shown that hair samples may be used to measure the levels of bisphenols, and that exposure to BPS may be greater than that to BPA in this area. The investigation also revealed that hair analysis is a useful approach for the biomonitoring of BPA and BPS levels in human organisms.
Keywords: bis(4-hydroxyphenyl)sulfone, Bisphenol A, Benzhydryl Compounds, Hair, hair analysis, Humans, Phenols, Pilot Projects, Poland
Background
Bisphenol A (BPA;2,2-bis(4-hydroxyphenyl)propane) is a synthetic compound that is widely utilized in numerous types of industry [1,2]. Primarily, BPA is utilized in the fabrication of plastics and it occurs in numerous everyday items, such as electronic equipment, auto parts, bottles, toys, clothes, and other items [2–4]. It has been found that BPA has the ability to leach from plastic items and seep into the environment; BPA has been found in water, soil, plants, and air throughout the world [5–7].
Moreover, it is known that BPA contained in items having contact with food and drinking water (such as bottles, food storage containers, internal layers of food cans, and water pipes) is especially dangerous for living organisms, because it penetrates into food and water; thereby entering into living organisms [2]. The digestive system is the main route of exposure for humans to BPA, but this compound also penetrates through the lungs, skin, and placenta during the prenatal period [2,3]. Previous observations have found the occurrence of BPA in different parts of the body, including blood serum, urine, breast milk, sperm, and placenta [3,8–11].
According to previous observations, BPA is an endocrine disruptor, showing various harmful activities [2,3]. Due to the fact that BPA is similar to estrogen, it acts on estrogenic receptors and causes disturbances in the functioning of various internal systems, including, among others, the nervous, immune, endocrine, reproductive, and gastrointestinal systems [12–14]. Moreover, correlations between the degree of exposure to BPA and increased risk of cancer, diabetes, hypertension, heart attack, and neurodegenerative diseases have also been reported [13–16]. Because of the strong harmful activity of BPA, numerous limitations in the utilization of this compound apply in many countries [17]. These restrictions mainly apply to items intended for newborns and children, as well as materials that come into contact with food and drinking water. “BPA-free” items, in which BPA is replaced with its analogues, are increasingly being produced [18].
One such BPA analogue, which is increasingly being used in the plastics industry is bisphenol S (BPS; 4-hydroxyphenyl sulfone). Similarly to BPA, BPS is present in various everyday objects and may penetrate into the environment, food, and living organisms [19–23]. Until recently, BPS has been considered less harmful to humans than BPA. However, recent studies have shown that BPS exhibits various harmful actions on living organisms, such as obesity; hypertension; endocrine disrupting and carcinogenic effects; neuro-, cyto- and genotoxicity, resulting in disturbances in reproduction; and other disorders [12,24,25]. Furthermore, some observations have found that the estrogenic properties of BPS are similar to or greater than such activity of BPA [26]. Knowledge concerning the factors that contribute to human exposure to BPS and the impact of this substance on human health is much more limited than that about BPA. However, existing investigations have described that human exposure to BPA is higher than to BPS [21,27,28], and therefore, BPA is often considered a more dangerous compound for humans.
With regard to the multifaceted harmful properties of BPA and BPS for humans, evaluation of exposure to these compounds is an important section of toxicology. Until recently, such monitoring was mainly based on the analysis of “classic” matrices, including blood serum and/or urine samples [29,30]. However, at present, the examination of hair specimens to identify the intensity of human exposure to bisphenols and other endocrine disruptors as an environmental pollutant is increasingly important [28,31–37]. This approach has the advantage that hair samples can be collected in an easier and less invasive way than blood serum. Moreover, hair samples can be easily stored for long periods and shipped over long distances.
Previous observations have found that the use of hair analysis to assess the degree of exposure to endocrine disruptors is viable and provides good results that, with regard to sensitivity, reliability, and reproducibility, are similar to results obtained during urine or blood serum analysis [28,31,38]. The utilization of hair examination in the evaluation of human exposure to bisphenols is a comparatively new approach. Nevertheless, in light of the previous studies, the analysis of hair samples seems to be a good alternative to other matrices for studies of human exposure to BPA and BPS [27,28,32,33].
Therefore, the aim of this study was to analyze the intensity of exposure of inhabitants of north-eastern Poland to BPA and BPS by evaluating 25 hair samples using liquid chromatography with a mass spectrometry technique (LC-MS) method. It is the first study in Poland in which analysis of hair samples was used to simultaneously monitor BPA and BPS. The investigation enriches the knowledge concerning human exposure to these compounds and the use of hair samples in such studies.
Material and Methods
SAMPLE COLLECTION:
Before sampling, all people included in the study were notified about the nature of the investigation, and all consented to the procedure. All procedures during the study were done in accordance with the guidelines of the Bioethical Committee at the School of Medicine of the University of Warmia and Mazury in Olsztyn (Poland) (agreement numbers: 27/2017 and 5/2021). The investigation was conducted in line with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments on humans. Subjects had to meet the following inclusion criteria to be eligible for the study: they had to be adult (over 18 years old), relatively healthy (without diagnosis of any long-term disease) and living in Olsztyn (a city of northeastern Poland). They could be either male or female, with any hair color. Subjects were randomly selected volunteers and during the present investigation no control group were formed because the aim of the study was to evaluate the levels of the industrial pollutants, bisphenol S and bisphenol A, in the hair samples in a representative group of residents of Olsztyn.
Hair specimens were gained from 25 residents (13 men and 12 women) of Olsztyn (a city inhabited by about 170 000 residents, located in northeastern Poland), on a voluntary basis. The age of volunteers ranged from 23 to 67 years (mean age 38.84±13.93). All volunteers were office workers or students and followed a varied diet including food products of plant and animal origin. The characteristics of the participants in the study are presented in Table 1. The method of sample collection has been described previously by Wojtkiewicz et al [33]. Hair specimens were gained from the location above the nape of the neck, as close as possible to the scalp. Right after cutting, hair specimens were put into aluminum foil sachets and kept in the dark at room temperature without any contact with plastics that could contain bisphenols.
REAGENTS:
The following reagents were used in the research: 1) BPA and BPS (both ≥98%), and ammonium acetate (≥98%) from Sigma-Aldrich (St. Louis MO, USA); 2) methanol (LC-MS grade) from Honeywell-Riedel de Haën (Wunstonfer Strasse, Seelze, Germany); 3) acetonitrile (LC-MS grade) from Fisher Chemical (Bishop Meadow Road, Loughborough, UK); 4) phenobarbital-d5 (IS) from Isotec Inc (Miamisburg, OH, USA); 5) ultrapure water, obtained using a Merck Direct-Q 3UV water purification system (Darmstadt, Germany).
EXTRACTION OF BPA AND BPS FROM HAIR:
Prior to extraction, hair specimens were rinsed twice with ultrapure water and twice in methanol. This rinsing was used to remove contaminants from the surface of the hair. Then, the hair specimens were dried at 50°C and cut into fragments with a length of several millimeters. The extraction was made according to the method described by Tzatzarakis et al [34]. In brief, 30–100 mg of each sample with 2×2 ml of methanol and 50 ng IS in glass screw tubes were subjected to extraction in an ultrasonic water bath for 2×2 h with periodic mixing with a vortex system. Then, the extracts were combined and evaporated to dryness under nitrogen steam at 35°C. After this, 100 μl of methanol was added to the residues, the solution was transferred into 2 ml vials with inserts for LC-MS analysis, and 10 μl of the solution was injected into the system.
INSTRUMENTATION:
An LC-MS system (Shimadzu, Kyoto, Japan, LC-MS 2010 EV) was used. For analyte separation, we used a Supelco Discovery column C18 (250 mm, 4.6 mm, 5 μm; Sigma-Aldrich, St. Louis, MO, USA) at a temperature of 30°C. The analysis was performed with a flow rate of 0.6 ml/min using a water gradient as solvent A and methanol as solvent B. To monitor the aforementioned substances, we used atmospheric pressure chemical ionization (APCI) and a quadrupole mass filter in negative selected ion monitoring (SIM) mode, with ions m/z 227.15, 259.1 for the BPA, 249.05, 285.05 for the BPS, and 236.05 for the IS. The interface, CDL, and heat block temperatures were set at 400°C, 200°C, and 200°C, respectively; the detector voltage at 1.5 kV; and the nebulizing gas flow at 2.5 L/min.
METHOD VALIDATION:
Analytical parameters to evaluate the efficacy of the methods were tested as follows. Standard solutions of the analytes were made in the following concentrations: 0, 50, 100, 250, and 500 ng/ml and their linearity was found to be 0.9993 for BPA and 0.9951 for BPS. Spiked sample analysis was performed for concentrations of 0, 10, 25, 50, 75, and 100 pg/mg for BPA and 0, 10, 25, 50, 75, 100, and 600 pg/mg for BPS with linearity at 0.9997 in the case of BPA and 0.9956 in the case of BPS.
Both limit of detection (LOD) and limit of quantification (LOQ) were evaluated using the signal to noise ratio. Three repeats of spiked samples (n=3) were used for the evaluation of the recovery, accuracy, and inter-day precision (%RSD) of the method. This was performed using levels 25, 50, 75, and 100 pg/mg for recovery and 10, 25, 50, 75, and 100 pg/mg for precision and accuracy (Table 2).
STATISTICAL ANALYSIS:
GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California, USA) was used for the statistical analysis. In the case of comparing 2 groups, the nonparametric Mann-Whitney test was used. The test power with the level of significance α=0.05 was 0.82. Data are presented as mean±standard deviation (SD). The differences were considered as statistically significant at
Results
During the present investigation, at least one of the bisphenols studied was found in each of the hair samples. In the case of both BPS and BPA, clearly visible differences in concentration levels between particular volunteers were noted (Table 3).
Generally, within all tested samples, BPS was detected in 96% of the human hair specimens studied and its concentration levels fluctuated from 13.4 pg/mg to as high as 1054.9 pg/mg with a mean value of 185.8±249.9 pg/mg. On the other hand, BPA was noted in a smaller percentage of the hair samples (72%). BPA concentration levels fluctuated from 3.6 pg/mg to 52.9 pg/mg with a mean value of 21.9±16.8 pg/mg. Aggregate data on levels of BPS and BPA in hair specimens are presented in Table 3 and Figure 1.
During the present investigation, comparisons of the samples according to sex, age, and hair coloring of the tested volunteers were made. In men, the mean levels of BPA were 23.26±17.53 pg/mg and BPS were 192±215.9 pg/mg. In women, the values were 19.70±16.59 pg/mg and 178.4±296 pg/mg for BPA and BPS, respectively. Although concentration levels of BPS and BPA were slightly higher in men than in women, these differences were not statistically significant (Figure 2). On the other hand, some differences in the frequency of the studied bisphenols were observed. In men, BPS was noted in 100% of samples, and BPA only in 84.62%. In women, both substances were found in a smaller number of samples, which was especially notable in the case of BPA. In women, BPS was observed in 91.67% of samples studied, and BPA solely in 58.33%.
Regarding the correlations between bisphenols and the age of volunteers, mean BPS concentration levels in younger volunteers (aged 22–35) were 116.6±123.1 pg/mg, while those in older volunteers (aged 45–47) were 282.6±346.3 pg/mg. BPA concentration levels were 26.84±20.16 pg/mg and 17.91±13.25 pg/mg in younger and older volunteers, respectively. Although BPS levels were somewhat higher and BPA levels were lower in older volunteers compared with younger volunteers, these differences were not statistically significant (Figure 3). In older volunteers, both BPS and BPA were detected in 90.91% of the specimens, while in younger volunteers, BPS was detected in all specimens studied (100%) but BPA was found in only 42.86% of the specimens.
In the comparison of BPS and BPA levels in hair specimens in volunteers with dyed and non-dyed hair, there were also no statistically significant differences observed between the 2 groups (Figure 4). Mean levels of bisphenols in persons with natural hair were 163.5±202.9 pg/mg for BPS and 22.86±16.78 pg/mg for BPA. In humans with dyed hair, the levels were 230.4±337 pg/mg and 19.92±18.16 pg/mg for BPS and BPA, respectively.
Discussion
The human hair specimens studied contained high levels of both BPA and BPS. Interestingly, no statistically significant differences in the level of bisphenols were found between volunteers of different age range, sex, and dyed vs natural-colored hair. However, the concentration of BPS in the tested samples was higher than that of BPA.
This investigation has confirmed that hair evaluation may be one of the ways for determining the intensity of exposure to BPA and BPS in humans. Until recently, urine samples have been the main matrix for research on this issue, and previous studies describing BPA and/or BPS in human hair were scarce (Table 4). Earlier observations on hair specimens and other matrices have indicated that exposure to BPA and BPS is extremely varied in different regions [31,41]. These differences are probably connected with industrialization and air pollution, which is supported by the fact that the rural population is usually less exposed to bisphenols than residents of large cities and shows lower levels of bisphenols when sampled [34].
A very important factor affecting human exposure to BPA and BPS is diet. It has been observed that limitation of foods from cans and plastic containers or fast foods results in a decrease in BPA and BPS levels in urine specimens within just 3 days [10]. Moreover, many other factors affecting BPA and BPS concentration levels in human organisms have been described, including, among others, the use of plastic cutlery and dishes, profession, lifestyle, and even the number of dental fillings [2,4,21,22]. It should be emphasized that many factors that affect human exposure to bisphenols have not yet been well defined. These factors cause significant variations in the levels of bisphenol among persons living in the same environment or eating similar food, which has been observed both in previous studies [28,31] and in the present investigation.
In recent years, hair analysis has been gaining importance as a method to determine the exposure of humans to organic environmental pollutants (Table 4). It has the advantages of easy material collection, as well as easy sample storage and shipping without the need for freezing. Simultaneously, in light of previous studies, results obtained via this method are sensitive and reliable, and accurately reflect human exposure to organic pollutants [38].
Of course, despite the advantages, hair studies as a way to determine exposure to bisphenols in humans have some ambiguities. First of all, substances may penetrate into the hair in 2 ways. The first route of exposure is ingestion, with the substance reaching the hair roots through the capillaries. The second route of exposure is penetration of the substance into the hair directly from the environment. These 2 sources mean that conclusions on whether the presence of a substance indicates external or internal exposure is not possible during hair analysis. Moreover, concentration levels of bisphenols in the urine may undergo short-term changes resulting from daily excretion, and changes in bisphenol concentration levels in the blood serum are connected with their penetration into internal organs and metabolism in the intestine and liver. Previous studies have shown that administration of BPA caused a significant increase in blood and/or urine concentration levels within a short time after exposure, followed by a decrease in these values [42]. In contrast to results from blood and urine, bisphenols accumulate in hair over a long time and are in the hair until the hair falls out. Accordingly, hair specimen evaluation is appropriate for determination of long-term exposure to bisphenols. However, it is not good for studying the influence of short-term factors, such as diet-induced changes. For such studies, urine or blood serum sample analysis would be a more suitable method.
Comparing the results obtained during the present study with those from previous investigations on human hair, significant differences can be seen (Table 4). First of all, high BPS concentration levels were visible in the present investigation. Interestingly, the median value determined in the present study is over 30 times higher than a value determined in China [27], about 28 times higher than one determined in Greece [32] and over 10 times higher than one determined in France [27]. The median concentration level of BPS observed in the present study is also 3 times higher than the value noted in inhabitants of Belgium, although the maximum BPS concentration level observed in Belgium amounted to 2298.0 ng/g and was higher than the maximum level noted in the present study (1054.9 ng/g) [28].
The reasons for such differences are unknown. Partially, they could arise from limitations in BPA utilization in the plastic industry and its replacement by BPS. However, such high BPS concentration levels strongly suggest that there are other (hitherto unspecified) factors influencing these values. Our results are all the more puzzling because the city of Olsztyn, where the samples were collected, is not a highly industrialized area, despite the existence of some branches of industry (including rubber, furniture, and food). The explanation of the obtained results is hindered by a lack of studies on BPS concentration levels in the environment, both in the Olsztyn area, and in the whole of Poland. Knowledge concerning BPS exposure in Poland is rudimentary and limited. To the authors’ knowledge, there are only 4 studies describing this substance in human blood [43], human breast milk [44,45], and raw and processed cow milk [23]. These investigations do not indicate a particularly high exposure of habitants of Poland to BPS, but they was performed in completely different regions of the country from the present study.
BPA levels observed in the present investigation were lower than levels found in other countries, although BPA is widely present in the environment in Poland [46–49]. Until now, only one study evaluating BPA in human hair has been performed in Poland [40]. This study reported median BPA concentration levels of 337.5 ng/g, so it is about 20 times higher than the value noted in the present study (Table 4). Such differences may be connected with BPA environmental pollution in various parts of Poland. (Until now, BPA levels in Olsztyn and its surroundings have not been studied).
In the present investigation, significant relationships between age or sex and BPA and BPS levels in the hair were not found. It should be emphasized that the results of previous studies on this issue are often contradictory. Some investigations have described higher concentration levels of bisphenols in children [35,50]. This could be explained by a lack of fully developed enzymes for bisphenol metabolism in children under the age of 8 years. Other observations have found higher BPA levels in older persons [31], which may be related to age-dependent changes and disorders in the digestive tract. A similar situation is noted in the case of sex-associated differences in concentration levels of bisphenols in the human body. Namely, some authors have reported higher bisphenol concentration levels in men, which is perhaps related to higher androgen levels [51], while others have described a higher concentration level of these substances in girls than in boys [35], and still others have not observed any sex differences [52]. Such discrepancies show that exact correlations between gender and/or age and bisphenol levels in humans is difficult, because exposure to these compounds is connected with numerous factors, including diet, lifestyle, residence, type of work, and even the number of dental fillings.
In the present investigation, no impact of artificial hair coloring on BPA and BPS levels was found, although, according to earlier studies, hair dying affects hair structure, integrity, and affinity for chemical compounds in the hair [54,55,58]. However, the results of this investigation are in line with earlier studies on human hair [28,36,37], indicating that hair analysis for the purpose of biomonitoring bisphenols in humans may be carried out using both natural and dyed hair.
The present study has some limitations. First of all, the number of investigated samples was relatively low. Although many previous findings concerning the evaluation of the level of harmful substances were performed on similar group size [29,31–33,36,38], a study of a larger population would give more representative results. Therefore, the present study should be considered as a pilot study and the monitoring of human exposure to bisphenols in Poland should be further investigated at a larger scale. Another limitation is the differentiation of samples in terms of hair length. It should be pointed out that in this kind of analysis it is very difficult to establish whether the results are an effect of external or internal exposure to the studied substance. Therefore, different lengths of hair samples may have had an influence on the obtained levels of BPA and BPS. Interestingly, there were no statistically significant differences between the groups of long and short hair, and the results obtained within each group had such high variation that comparison of these categories was not considered for this report. However, the present findings did show a high concentration of both BPA and BPS in the tested samples. Therefore, future analyses of exposure to these bisphenols on a larger scale may wish to include comparisons of different hair length categories.
Conclusions
The present work represents the first biomonitoring of bisphenols in hair in residents of Poland. Additionally, for the first time, the levels of BPS in human hair specimens were found to be higher than those of BPA. This indicates that BPS is an important environmental pollutant, and human exposure to this substance is high. The reason for such high levels of BPS is for now unknown, because the body of knowledge regarding BPS occurrence in the Polish environment is nonexistent. In our study, no statistically significant differences in bisphenol concentration according to age, gender, and/or artificial hair coloring were found. This work may be treated as a preliminary investigation to further studies exploring the occurrence of bisphenols in Poland. Nevertheless, the present work has shown that hair examination can be a valid way of monitoring longer-term bisphenol exposure in humans.
Figures
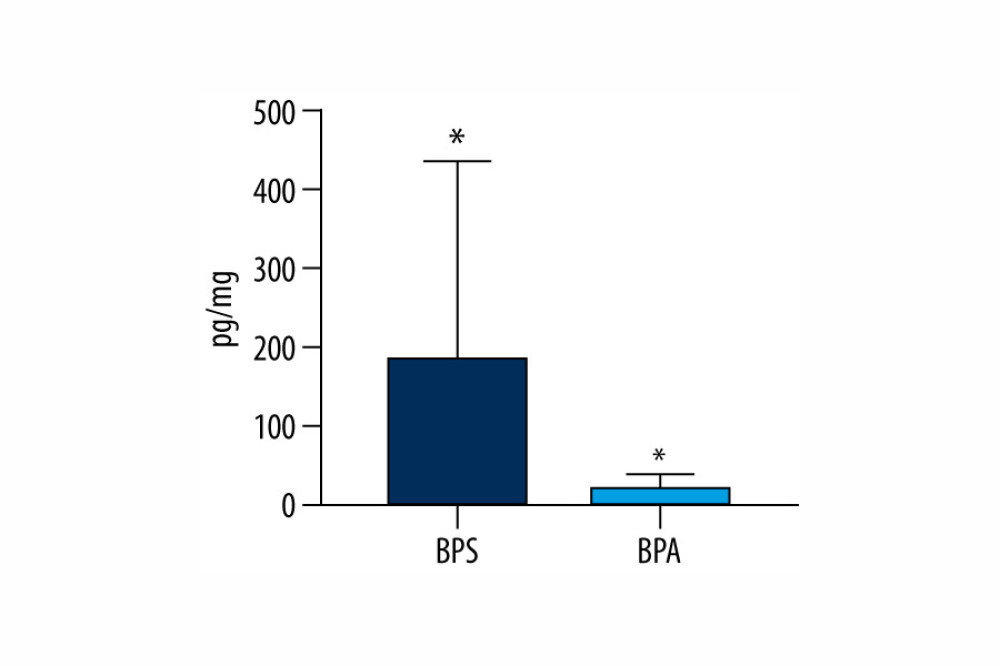 Figure 1. Mean concentration levels (±SD) of bisphenol A (BPA) and bisphenol S (BPS) in human hair samples. Statistically significant differences (P≤0.05) are marked with *. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA).
Figure 1. Mean concentration levels (±SD) of bisphenol A (BPA) and bisphenol S (BPS) in human hair samples. Statistically significant differences (P≤0.05) are marked with *. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA). 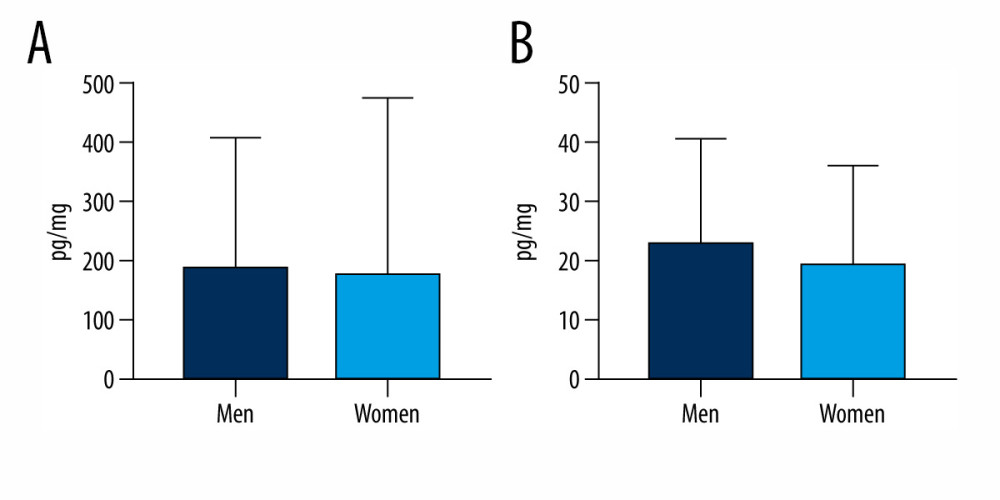 Figure 2. Mean concentration levels (±SD) of (A) bisphenol S and (B) bisphenol A in hair samples of men and women. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA).
Figure 2. Mean concentration levels (±SD) of (A) bisphenol S and (B) bisphenol A in hair samples of men and women. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA). 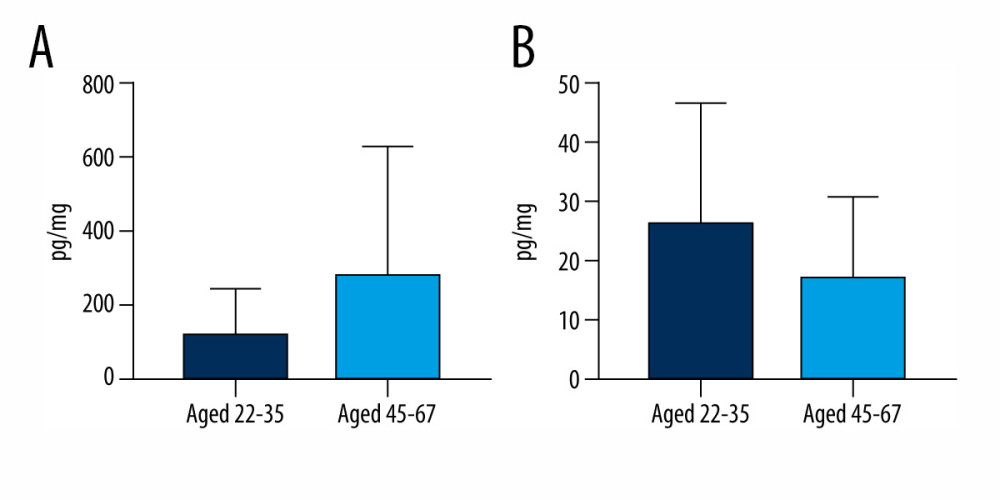 Figure 3. Mean concentration levels (±SD) of (A) bisphenol S and (B) bisphenol A in persons between the ages of 22–35 and 45–67. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA).
Figure 3. Mean concentration levels (±SD) of (A) bisphenol S and (B) bisphenol A in persons between the ages of 22–35 and 45–67. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA). 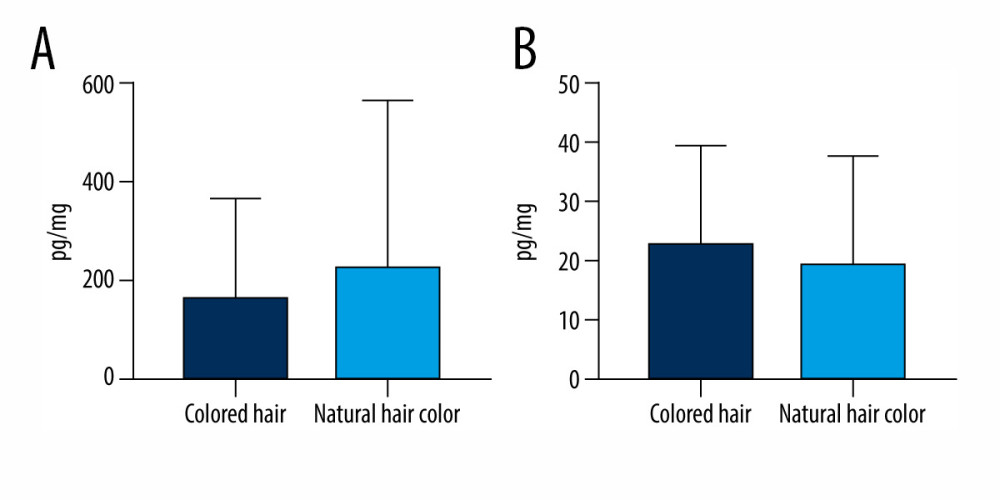 Figure 4. Mean concentration levels (±SD) of (A) bisphenol S and (B) bisphenol A in persons with artificially colored hair and natural-colored hair. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA).
Figure 4. Mean concentration levels (±SD) of (A) bisphenol S and (B) bisphenol A in persons with artificially colored hair and natural-colored hair. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA). Tables
Table 1. Characterization (demographic features) of volunteers taking part in the study.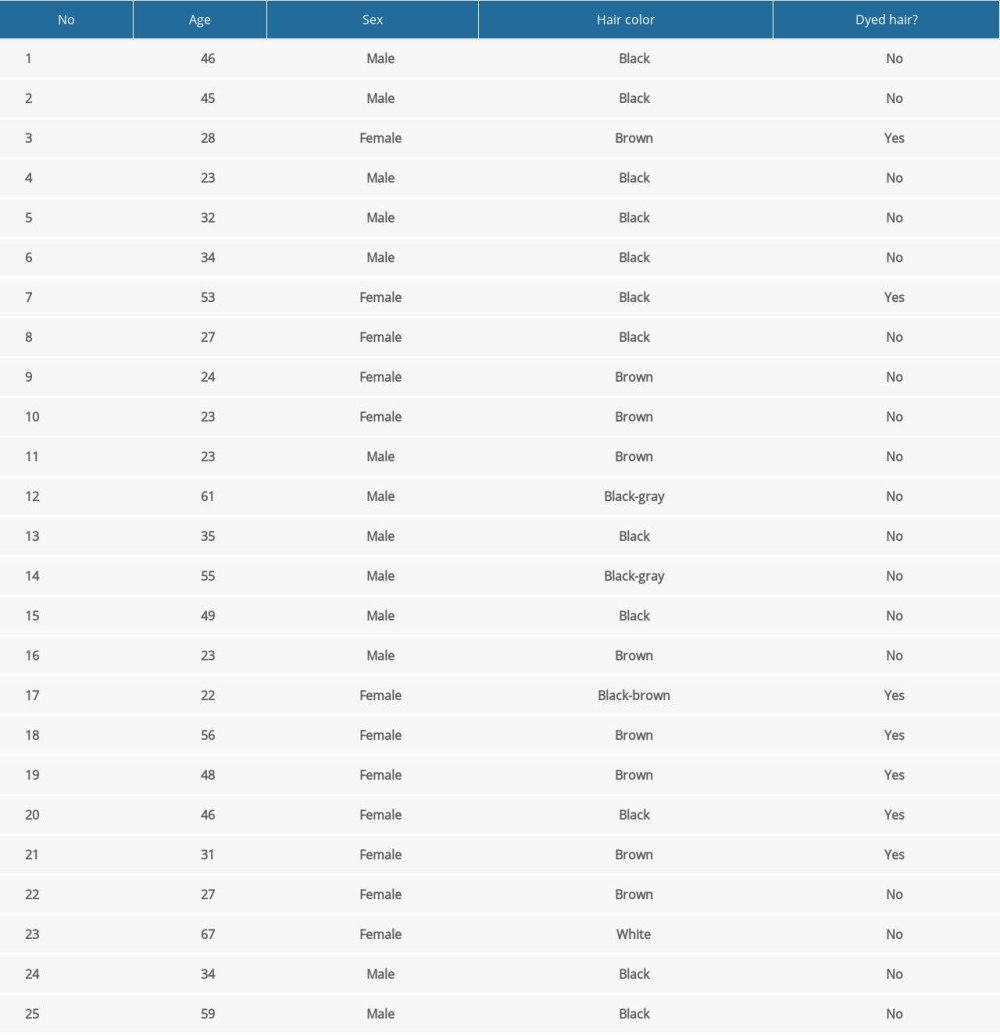 Table 2. Validation parameters of the applied methodology.
Table 2. Validation parameters of the applied methodology.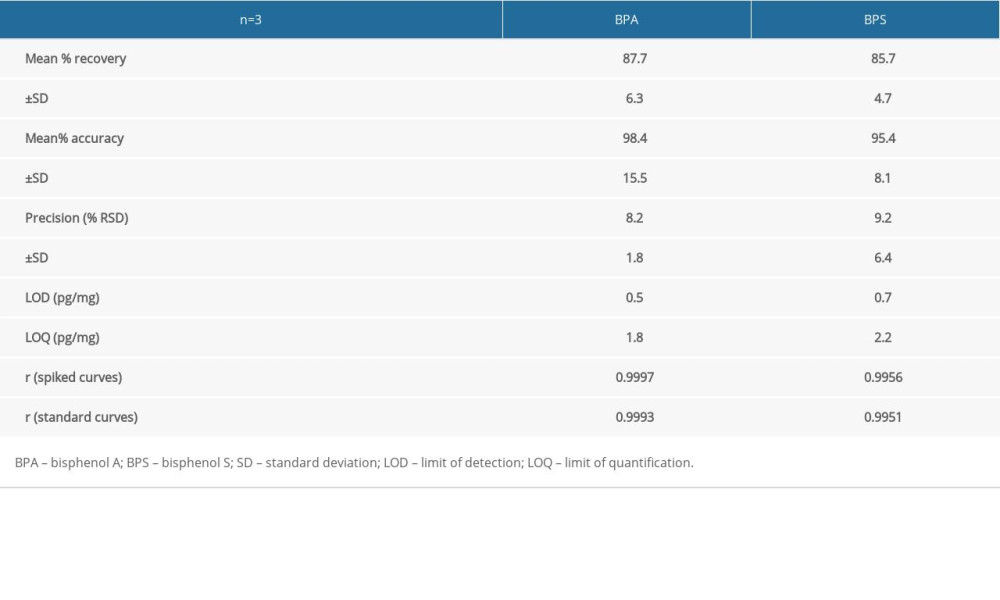 Table 3. Cumulative data concerning bisphenol A and bisphenol S concentration levels (pg/mg) in hair samples, obtained in the present study.
Table 3. Cumulative data concerning bisphenol A and bisphenol S concentration levels (pg/mg) in hair samples, obtained in the present study.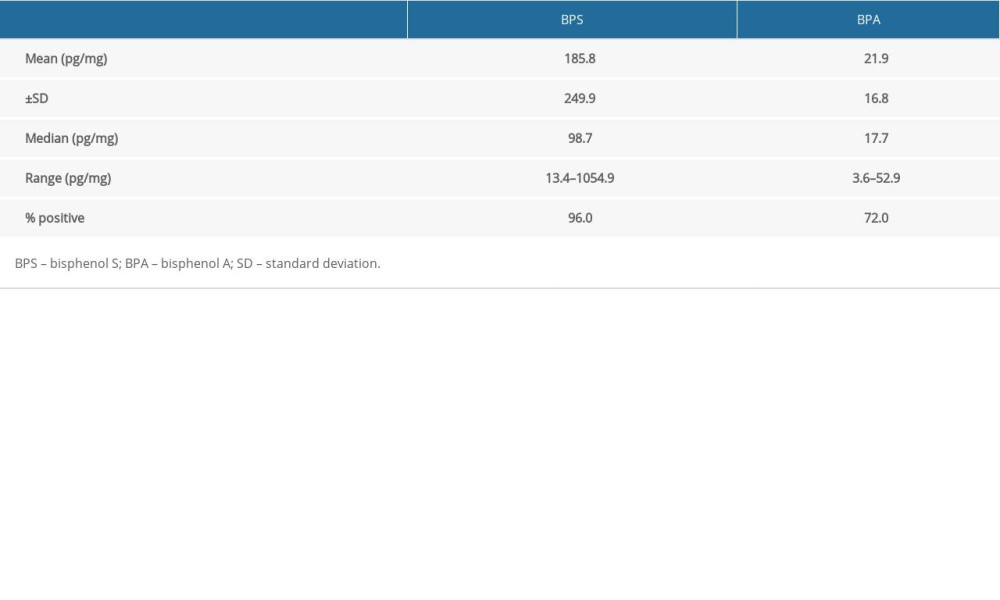 Table 4. Biomonitoring studies of bisphenol A and bisphenol S in human hair.
Table 4. Biomonitoring studies of bisphenol A and bisphenol S in human hair.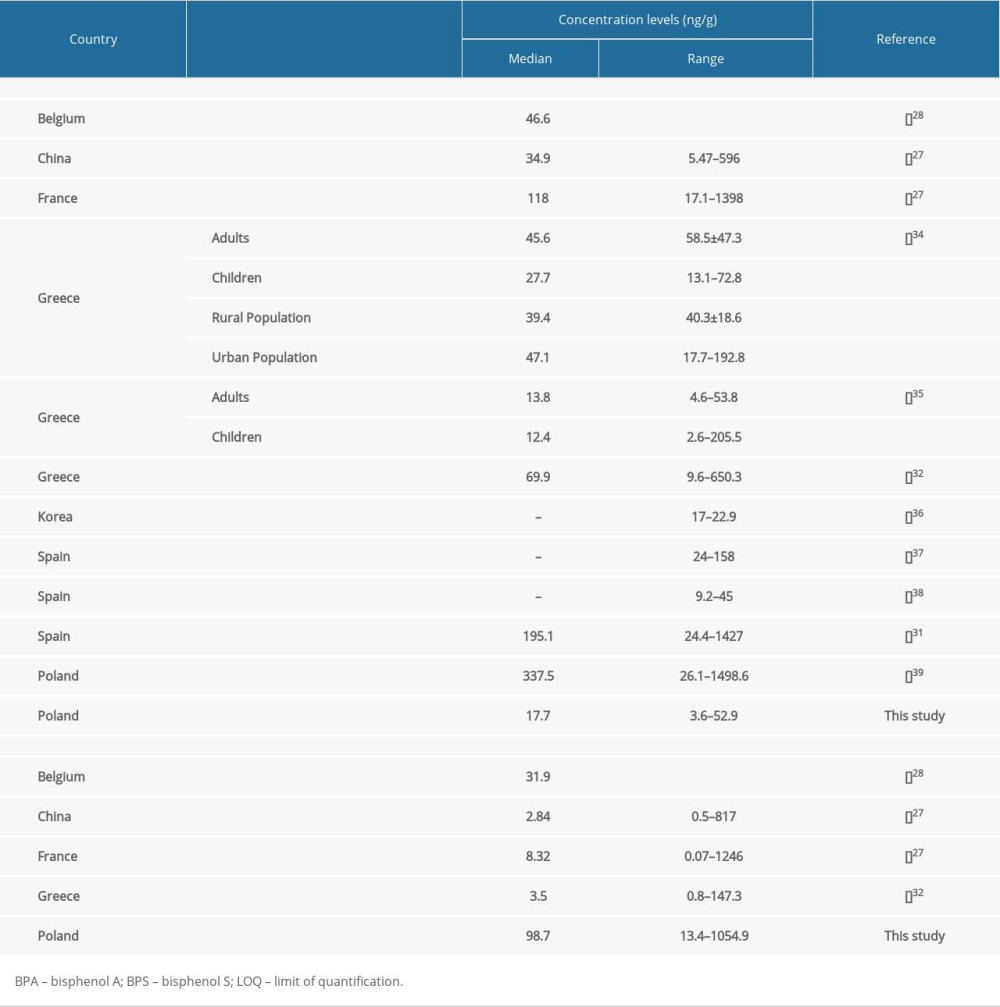
References
1. Ide Y, Kagawa N, Itakura M, Effective and selective bisphenol A synthesis on a layered silicate with spatially arranged sulfonic acid: ACS Appl Mater Interfaces, 2012; 4; 2186-91
2. Michałowicz J, Bisphenol A – sources, toxicity and biotransformation: Environ Toxicol Pharmacol, 2014; 37; 738-58
3. Vandenberg LN, Hauser R, Marcus M, Human exposure to bisphenol A (BPA): Reprod Toxicol, 2007; 24; 139-77
4. Löfroth M, Ghasemimehr M, Falk A, Bisphenol A in dental materials – existence, leakage, and biological effects: Heliyon, 2019; 5; e01711
5. Abraham A, Chakraborty P, A review on sources and health impacts of bisphenol A: Rev Environ Health, 2020; 35; 201-10
6. Gonsiorowski A, Mourikes VE, Flaws JA, Endocrine disruptors in water and their effects on the reproductive system: Int J Mol Sci, 2020; 21; 1929
7. Xu Y, Hu A, Li Y, Determination and occurrence of bisphenol A and thirteen structural analogs in soil: Chemosphere, 2021; 277; 130232
8. Fernández MF, Arrebola JP, Jiménez-Díaz I, Bisphenol A and other phenols in human placenta from children with cryptorchidism or hypospadias: Reprod Toxicol, 2016; 59; 89-95
9. Radwan M, Wielgomas B, Dziewirska E, Urinary bisphenol A levels and male fertility: Am J Mens Health, 2018; 12; 2144-51
10. Kim S, Lee I, Lim JE, Dietary contribution to body burden of bisphenol A and bisphenol S among mother-children pairs: Sci Total Environ, 2020; 744; 140856
11. Kim JH, Kim D, Moon SM, Associations of lifestyle factors with phthalate metabolites, bisphenol A, parabens, and triclosan concentrations in breast milk of Korean mothers: Chemosphere, 2020; 249; 126149
12. Rochester JR, Bolden AL, Bisphenol S and F: A Systematic review and comparison of the hormonal activity of bisphenol A substitutes: Environ Health Perspect, 2015; 123; 643-50
13. Konieczna A, Rutkowska A, Rachoń D, Health risk of exposure to Bisphenol A (BPA): Rocz Panstw Zakl Hig, 2015; 66; 5-11
14. Priego AR, Parra EG, Mas S, Bisphenol A modulates autophagy and exacerbates chronic kidney damage in mice: Int J Mol Sci, 2021; 22; 7189
15. Pérez-Bermejo M, Mas-Pérez I, Murillo-Llorente MT, The role of the bisphenol A in diabetes and obesity: Biomedicines, 2021; 9; 666
16. Rebolledo-Solleiro D, Castillo Flores LY, Solleiro-Villavicencio H, Impact of BPA on behavior, neurodevelopment and neurodegeneration: Front Biosci (Landmark Ed), 2021; 26; 363-400
17. Barraza L, A new approach for regulating bisphenol A for the protection of the public’s health: J Law Med Ethics, 2013; 41(Suppl 1); 9-12
18. Usman A, Ahmad M, From BPA to its analogues: Is it a safe journey?: Chemosphere, 2016; 158; 131-42
19. Zhou J, Chen XH, Pan SD, Contamination status of bisphenol A and its analogues (bisphenol S, F and B) in foodstuffs and the implications for dietary exposure on adult residents in Zhejiang Province: Food Chem, 2019; 294; 160-70
20. Wan Y, Xia W, Yang S, Spatial distribution of bisphenol S in surface water and human serum from Yangtze River watershed, China: Implications for exposure through drinking water: Chemosphere, 2018; 199; 595-602
21. Wu LH, Zhang XM, Wang F, Occurrence of bisphenol S in the environment and implications for human exposure: A short review: Sci Total Environ, 2018; 615; 87-98
22. Bousoumah R, Leso V, Iavicoli I, Biomonitoring of occupational exposure to bisphenol A, bisphenol S and bisphenol F: A systematic review: Sci Total Environ, 2021; 783; 146905
23. Frankowski R, Grześkowiak T, Czarczyńska-Goślińska B, Occurrence and dietary risk of bisphenols and parabens in raw and processed cow’s milk: Food Addit Contam Part A Chem Anal Control Expo Risk Assess, 2021; 26; 1-14
24. Fouyet S, Olivier E, Leproux P, Bisphenol A, bisphenol F, bisphenol S: The bad and the ugly. Where is the good?: Life (Basel), 2021; 11; 314
25. Xiao X, Zhang X, Bai J: Food Chem, 2021; 339; 127813
26. Thoene M, Dzika E, Gonkowski S, Bisphenol S in food causes hormonal and obesogenic effects comparable to or worse than bisphenol A: A literature review: Nutrients, 2020; 12; 532
27. Peng FJ, Hardy EM, Béranger R, Human exposure to PCBs, PBDEs and bisphenols revealed by hair analysis: A comparison between two adult female populations in China and France: Environ Pollut, 2020; 267; 115425
28. Claessens J, Pirard C, Charlier C, Determination of contamination levels for multiple endocrine disruptors in hair from a non-occupationally exposed population living in Liege (Belgium): Sci Total Environ, 2021; 815; 152734
29. Hines EP, Mendola P, von Ehrenstein OS, Concentrations of environmental phenols and parabens in milk, urine and serum of lactating North Carolina women: Reprod Toxicol, 2015; 54; 120-28
30. Vorkamp K, Castaño A, Antignac JP, Biomarkers, matrices and analytical methods targeting human exposure to chemicals selected for a European human biomonitoring initiative: Environ Int, 2021; 146; 106082
31. Martín J, Santos JL, Aparicio I, Exposure assessment to parabens, bisphenol A and perfluoroalkyl compounds in children, women and men by hair analysis: Sci Total Environ, 2019; 695; 133864
32. Katsikantami I, Tzatzarakis MN, Karzi V, Biomonitoring of bisphenols A and S and phthalate metabolites in hair from pregnant women in Crete: Sci Total Environ, 2019; 712; 135651
33. Wojtkiewicz J, Tzatzarakis M, Vakonaki E, Evaluation of human exposure to parabens in north eastern Poland through hair sample analysis: Sci Rep, 2021; 11(1); 23673
34. Tzatzarakis MN, Vakonaki E, Kavvalakis MP, Biomonitoring of bisphenol A in hair of Greek population: Chemosphere, 2017; 118; 336-41
35. Karzi V, Tzatzarakis MN, Vakonaki E, Biomonitoring of bisphenol A, triclosan and perfluorooctanoic acid in hair samples of children and adults: J Appl Toxicol, 2018; 38; 1144-52
36. Lee C, Kim CH, Kim S, Simultaneous determination of bisphenol A and estrogens in hair samples by liquid chromatography-electrospray tandem mass spectrometry: J Chromatogr, 2017; B 1058; 8-13
37. Martín J, Santos JL, Aparicio I, Analytical method for biomonitoring of endocrine-disrupting compounds (bisphenol A, parabens, perfluoroalkyl compounds and a brominated flame retardant) in human hair by liquid chromatography-tandem mass spectrometry: Anal Chim Acta, 2016; 945; 95-101
38. Alves A, Jacobs G, Vanermen G, New approach for assessing human perfluoroalkyl exposure via hair: Talanta, 2015; 144; 574-83
39. Rodríguez-Gómez R, Martín J, Zafra-Gómez A, Biomonitoring of 21 endocrine disrupting chemicals in human hair samples using ultra-high performance liquid chromatography tandem mass spectrometry: Chemosphere, 2017; 168; 676-84
40. Nehring I, Staniszewska M, Falkowska L: Arch Environ Contam Toxicol, 2017; 72; 552-61
41. Huang RP, Liu ZH, Yin H, Bisphenol A concentrations in human urine, human intakes across six continents, and annual trends of average intakes in adult and child populations worldwide: A thorough literature review: Sci Total Environ, 2018; 626; 971-81
42. Thayer KA, Doerge DR, Hunt D, Pharmacokinetics of bisphenol A in humans following a single oral administration: Environ Int, 2015; 83; 107-15
43. Komarowska MD, Grubczak K, Czerniecki J, Identification of the bisphenol A (BPA) and the two analogues BPS and BPF in Cryptorchidism: Front Endocrinol (Lausanne), 2021; 12; 694669
44. Tuzimski T, Pieniążek D, Buszewicz G, QuEChERS-based extraction procedures for the analysis of bisphenols S and A in breast milk samples by LC-QqQ-MS: J AOAC, 2018; 102; 23-32
45. Tuzimski T, Szubartowski S, Gadzała-Kopciuch R, Comparison of DAD and FLD detection for identification of selected bisphenols in human breast milk samples and their quantitative analysis by LC-MS/MS: J AOAC, 2020; 103; 1029-42
46. Staniszewska M, Falkowska L, Grabowski P, Bisphenol A, 4-tert-octylphenol, and 4-nonylphenol in the Gulf of Gdańsk (Southern Baltic): Arch Environ Contam Toxicol, 2014; 67; 335-47
47. Czarczyńska-Goślińska B, Zgoła-Grześkowiak A, Jeszka-Skowron M, Detection of bisphenol A, cumylphenol and parabens in surface waters of Greater Poland Voivodeship: J Environ Manage, 2017; 204; 50-60
48. Kaleniecka A, Zarzycki PK, Analysis of selected endocrine disrupters fraction including bisphenols extracted from daily products, food packaging and treated wastewater using optimized solid-phase extraction and temperature-dependent inclusion: Chromatography Mol, 2019; 24; 1285
49. Bodziach K, Staniszewska M, Falkowska L, Gastrointestinal and respiratory exposure of water birds to endocrine disrupting phenolic compounds: Sci Total Environ, 2021; 754; 142435
50. Calafat AM, Ye XY, Wong LY, Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003–2004: Environ Health Perspect, 2008; 116; 39-44
51. Takeuchi T, Tsutsumi O, Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels: Biochem Biophys Res Commun, 2002; 291; 76-78
52. Mahalingaiah S, Meeker JD, Pearson KR, Temporal variability and predictors of urinary bisphenol A concentrations in men and women: Environ Health Perspect, 2008; 116; 173-78
53. Appenzeller BMR, Tsatsakis AM, Hair analysis for biomonitoring of environmental and occupational exposure to organic pollutants: State of the art, critical review and future needs: Toxicol Lett, 2012; 210; 119-40
54. Covaci A, Tutudaki M, Tsatsakis AM, Hair analysis: Another approach for the assessment of human exposure to selected persistent organochlorine pollutants: Chemosphere, 2002; 46; 413-18
55. Gerace E, Veronesi A, Martra G, Study of cocaine incorporation in hair damaged by cosmetic treatments: Forensic Chem, 2017; 3; 69-73
Figures
 Figure 1. Mean concentration levels (±SD) of bisphenol A (BPA) and bisphenol S (BPS) in human hair samples. Statistically significant differences (P≤0.05) are marked with *. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA).
Figure 1. Mean concentration levels (±SD) of bisphenol A (BPA) and bisphenol S (BPS) in human hair samples. Statistically significant differences (P≤0.05) are marked with *. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA). Figure 2. Mean concentration levels (±SD) of (A) bisphenol S and (B) bisphenol A in hair samples of men and women. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA).
Figure 2. Mean concentration levels (±SD) of (A) bisphenol S and (B) bisphenol A in hair samples of men and women. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA). Figure 3. Mean concentration levels (±SD) of (A) bisphenol S and (B) bisphenol A in persons between the ages of 22–35 and 45–67. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA).
Figure 3. Mean concentration levels (±SD) of (A) bisphenol S and (B) bisphenol A in persons between the ages of 22–35 and 45–67. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA). Figure 4. Mean concentration levels (±SD) of (A) bisphenol S and (B) bisphenol A in persons with artificially colored hair and natural-colored hair. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA).
Figure 4. Mean concentration levels (±SD) of (A) bisphenol S and (B) bisphenol A in persons with artificially colored hair and natural-colored hair. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA). Tables
 Table 1. Characterization (demographic features) of volunteers taking part in the study.
Table 1. Characterization (demographic features) of volunteers taking part in the study. Table 2. Validation parameters of the applied methodology.
Table 2. Validation parameters of the applied methodology. Table 3. Cumulative data concerning bisphenol A and bisphenol S concentration levels (pg/mg) in hair samples, obtained in the present study.
Table 3. Cumulative data concerning bisphenol A and bisphenol S concentration levels (pg/mg) in hair samples, obtained in the present study. Table 4. Biomonitoring studies of bisphenol A and bisphenol S in human hair.
Table 4. Biomonitoring studies of bisphenol A and bisphenol S in human hair. Table 1. Characterization (demographic features) of volunteers taking part in the study.
Table 1. Characterization (demographic features) of volunteers taking part in the study. Table 2. Validation parameters of the applied methodology.
Table 2. Validation parameters of the applied methodology. Table 3. Cumulative data concerning bisphenol A and bisphenol S concentration levels (pg/mg) in hair samples, obtained in the present study.
Table 3. Cumulative data concerning bisphenol A and bisphenol S concentration levels (pg/mg) in hair samples, obtained in the present study. Table 4. Biomonitoring studies of bisphenol A and bisphenol S in human hair.
Table 4. Biomonitoring studies of bisphenol A and bisphenol S in human hair. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








