07 March 2024: Clinical Research
Changes in Spectrum of Respiratory Pathogen Infections and Disease Severity Among Children in Hohhot, China: Impact of COVID-19 Prevention Measures
Yan-Zi Gan1ABCDG, Peng Yang2BCD, Rui Liu3CE, Yan-Hai Wang1B, Yu-Wei Hu1B, Yang YangDOI: 10.12659/MSM.942845
Med Sci Monit 2024; 30:e942845
Abstract
BACKGROUND: This retrospective study evaluated the effects of specific COVID-19 preventive measures, including the use of medical masks, nucleic acid testing, and patient isolation, on respiratory infections, disease severity, and seasonal patterns among children in Hohhot, located in northern China. Understanding these alterations is pivotal in developing effective strategies to handle pediatric respiratory infections within the context of continuous public health initiatives.
MATERIAL AND METHODS: At the First Hospital of Hohhot, throat swabs were collected from 605 children with community-acquired respiratory between January 2022 and March 2023 for pathogen infection spectrum detection using microarray testing.
RESULTS: Among the patients, 56.03% were male, and their average age was 3.45 years. SARS-CoV-2 infections were highest between October 2022 and January 2023. Influenza A peaked in March 2023, and other pathogens such as respiratory syncytial virus and influenza B virus disappeared after December 2022. The proportion of mixed infections was 41.94% among SARS-CoV-2 patients, while other pathogens had mixed infection rates exceeding 57.14%. Before December 2022, the mean WBC count for Streptococcus pneumoniae and Haemophilus influenzae was 8.83×10⁹/L, CRP was 18.36 mg/L, and PCT was 1.11 ng/ml. After December 2022, these values decreased significantly. Coughing, difficulty breathing, running nose, and lower respiratory tract infection diagnoses decreased in December 2022, except for SARS-CoV-2 infections.
CONCLUSIONS: SARS-CoV-2 peaked around November 2022, influenza A peaked in March 2023, and other pathogens like respiratory syncytial virus and influenza B virus were greatly reduced after December 2022. Inflammatory markers and respiratory symptoms decreased after December 2022, except for SARS-CoV-2.
Keywords: biomarkers, Microarray Analysis, Epidemiology, Community-Acquired Infections
Background
Acute respiratory infections (ARIs) encompass a broad range of infections affecting the respiratory system, including the upper and lower respiratory tracts. These infections can be caused by various pathogens, such as viruses, bacteria, and fungi. Viral pathogens associated with ARIs include influenza A virus (IAV), respiratory syncytial virus (RSV), adenovirus (ADV), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), rhinoviruses (RV), and metapneumovirus (MPV) [1,2]. Bacterial pathogens, such as
Historically, influenza pandemics, such as the 1918 H1N1 influenza pandemic and the 1968 H3N2 “Hong Kong influenza” pandemic, have resulted in millions of deaths worldwide [8,9]. The recent COVID-19 pandemic caused by SARS-CoV-2 has had a profound global impact, with 765 000 000 confirmed cases and 6 927 000 deaths [10]. Several studies have demonstrated a decrease in the incidence and severity of respiratory infections during the implementation of SARS-CoV-2 control measures. The Chinese government implemented various non-pharmaceutical interventions (NPIs) during the COVID-19 outbreak, including banning social gatherings, mandating mask usage, and promoting remote work. Additionally, they postponed the spring 2020 semester for primary and middle schools, leading to a complete suspension of in-person classes. Students had to attend online courses from home until schools reopened in early June 2020. The scale and scope of these measures surpassed any previous NPIs [11]. For example, a study conducted in China reported a significant reduction in the number of influenza cases and other respiratory infections in children during the SARS-CoV-2 outbreak due to stringent control measures [12]. Similarly, a study from the Netherlands showed a decline in RSV infections during the SARS-CoV-2 pandemic, likely attributable to preventive measures in place [13]. Furthermore, research conducted during the SARS-CoV-2 pandemic has shown a decrease in cases of influenza and other respiratory viruses due to NPIs [14–17]. From 2023, China’s Non-National Immunization Program (NIP) has defined 13 routine vaccines, including those targeting respiratory-related diseases such as tuberculosis, pertussis, diphtheria, and measles [18]. Theinitial immunization schedule is within 8 months, and these vaccines are provided free of charge to the entire population, significantly reducing the incidence of related diseases. Additionally, several non-NIP vaccines are also commonly used for respiratory disease prevention, including Pneumococcal Conjugate Vaccine 13 (PCV13), SARS-CoV-2,
Influenza virus has been detected in Beijing, China, located approximately 400 miles away from Hohhot, China. The rate of influenza-like illness in the 2015–2016 season was 2.2%, increasing to 6.9% in the 2017–2018 season [19]. From 2019 to 2021, the Chinese National Influenza Surveillance Network reported a significant decline in the number of confirmed cases of IAV in northern China, with a decrease from 9642 to 11. The predominant strains during this period were primarily H1N1 and H3N2 [20]. In our previous reports, we presented the dynamics of blood biomarkers among patients with ARIs from 2015 to 2019 in Hohhot [21,22]. During that period, there were fewer cases of IAV and no instances of SARS-CoV-2 infection. In the 2019–2020 season, the circulation of influenza viruses in Hohhot, specifically H1N1 (n=1; 0.38%), was significantly lower compared to the seasons before the pandemic [21]. However, despite the decline in respiratory infections, reports of co-infection with influenza and SARS-CoV-2 have been documented in some countries. For instance, the Centers for Disease Control and Prevention (CDC) in the United States reported cases of individuals being simultaneously infected with IAV and SARS-CoV-2 during the autumn of 2020 [23]. These co-infection cases exhibit overlapping symptoms, leading to numerous instances of misdiagnosis or underdiagnosis in various countries [24,25].
This study evaluated respiratory pathogens in 605 untreated children with respiratory diseases prior to hospitalization at the First Hospital of Hohhot, situated in northern China, from January 2022 to March 2023. We analyzed the impact of these measures on pediatric respiratory infections, case severity, and seasonal pattern changes. By conducting a comparative analysis of data before and after implementation of COVID-19 control policies in Hohhot, we aim to provide insights into the alterations induced by these measures amid the ongoing COVID-19 situation.
Material and Methods
STUDY DESIGN AND PATIENT POPULATION:
This retrospective study was conducted at the First Hospital of Hohhot in China and focused on children with community-acquired respiratory infections who had not received any anti-infective treatment from January 2022 to March 2023. Specialists in respiratory tract infections examined and preliminarily diagnosed these children at the hospital’s outpatient department. Throat swabs and blood samples were collected from each child. The diagnostic criteria for respiratory tract infection in children included abnormalities in blood biochemical indicators (eg, white blood cell count, C-reactive protein, procalcitonin, proprotein, or erythrocyte sedimentation rate) and the presence of respiratory symptoms (eg, fever, cough, convulsion, difficulty breathing, throat discomfort, vomiting and diarrhea, runny nose). Acute respiratory tract infection (ARTI) cases were further categorized into upper respiratory tract infections (URTI) and lower respiratory tract infections (LRTI). Children hospitalized with diagnoses of bronchitis and pneumonia were classified as LRTI, including lobular pneumonia and lobar pneumonia [26]. Auxiliary imaging techniques were used to assist in diagnosing potential lower respiratory tract infections, and all 605 patients received treatment after confirmation of diagnosis in the hospital. Based on clinical assessments using symptoms and signs, clinical examination, and laboratory tests, 605 confirmed ARTI cases underwent microarray-based testing of throat swab samples. This method revealed a diverse range of microorganisms in the upper respiratory tract. All pathogens detected in this study were transmitted through the respiratory tract but did not necessarily cause significant respiratory infections. While this approach does not definitively identify a single causative agent, it allows consideration of potential co-infections or colonization of multiple pathogens throughout the patient’s entire respiratory tract, contributing to the complexity of observed clinical presentations. This comprehensive understanding is crucial for understanding the intricate dynamics of respiratory infections in children.
SPECIMEN COLLECTION:
The research protocol and data collection methods were approved by the Ethics Committee of the First Hospital of Hohhot (approval number: IRB2022011), and consent was obtained from the guardians of the children. Throat swabs were collected from the patients after obtaining parental or guardian consent. Blood samples were collected upon admission from the children using anticoagulant tubes containing EDTA-K2.
BLOOD BIOMARKER ANALYSIS:
Within 12 hours of hospital admission, WBC was assessed using SYSMEX-800i hematology analyzers (Sysmex, China). The serum levels of PCT and CRP were measured using a Hitachi 7600 analyzer (Roche, Basel, Switzerland). The normal range for WBC was defined as ≥3.5 or ≤9.5×109/L, while the normal values for CRP and PCT were ≤6.0 mg/L and ≤0.5 ng/ml, respectively. Due to limitations in the testing facility, the differential count of leukocytes was not recorded for many patients.
PATHOGEN DETECTION:
A total of 34 respiratory pathogen detection kits (Microarray) (Cat number: 0006, Kunming Huan ji Biochip Industry Co., Ltd) were used to identify pathogens in the throat swab samples. The microarray was scanned using a Microarray Scanner (Axon Gene Pix 4100A scanner, Union City, CA), and the microarray data was analyzed using GenePix Pro software for scanner control and image analysis [27]. The microarray kit employed in this study could detect and distinguish various pathogens, which were grouped into 2 main categories: common respiratory pathogens and less common ones that can cause respiratory infections in individuals with compromised or weakened immune systems or exhibit clinical symptoms similar to respiratory infections. Common respiratory tract infection pathogens include Streptococcus pneumoniae (S. pneumoniae), Haemophilus influenzae (H. influenzae), Mycoplasma pneumoniae (M. pneumoniae), Streptococcus pyogenes (Group A Streptococcus, GAS), Chlamydophila pneumoniae (C. pneumoniae), Bordetella pertussis (B. pertussis), Klebsiella pneumoniae (K. pneumoniae), Acinetobacter baumannii (A. baumannii), Staphylococcus aureus (S. aureus), Legionella pneumophila (L. pneumophila), Pseudomonas aeruginosa (P. aeruginosa), influenza A virus (IAV), influenza B virus (IBV), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), adenovirus (ADV) subtypes 1, 2, 3, 4, 5, 6, 7, 8, 14, 19, 21, 25, parainfluenza virus (PIV) subtypes 1, 2, 3, 4, respiratory syncytial virus (RSV) subtypes A and B, rhinovirus (RV), and metapneumovirus (MPV). Less common pathogens that can infect the respiratory tract of individuals with immunodeficiency or exhibit clinical symptoms resembling respiratory infections include Escherichia coli (E. coli), Streptococcus agalactiae (Group B Streptococcus, GBS), Neisseria meningitidis (N. meningitidis), Candida albicans (C. albicans), Campylobacter jejuni (C. jejuni), Epstein-Barr Virus (EBV), and cytomegalovirus (CMV) [28,29]. Epstein-Barr virus (EBV) and cytomegalovirus (CMV) are transmitted through the respiratory tract but do not infect the respiratory tract, occasionally causing symptoms such as fever and sore throat, leading to confusion in diagnosing respiratory tract infections [30,31].
CLINICAL DATA COLLECTION AND DEFINITIONS:
Various clinical data, including basic patient information (eg, age, sex, and admission time) and clinical diagnosis records (eg, body temperature, symptoms, and disease type) were collected and compiled into electronic documents. These documents were reviewed and validated by a team of pediatricians. For access to the original documents, please contact the authors via email. The electronic documents also recorded blood biochemical indicators and types of pathogenic infections.
STATISTICAL ANALYSES:
Statistical significance was determined using GraphPad Prism 7.0 (LLC Corp.) and Origin 2021 software (OriginLab Corp.). For comparisons among multiple groups, one-way analysis of variance (ANOVA) followed by Tukey’s test was performed. To assess significance between 2 groups, the
ETHICS APPROVAL AND CONSENT TO PARTICIPATE:
This research protocol and data collection methods followed standard operating procedures and were approved by the Ethics Committee of the First Hospital of Hohhot (approved number: IRB2022011). Informed consent was obtained from the legal guardians of all the participants. All methods were carried out in accordance with the Helsinki Declaration.
Results
PATIENT CHARACTERISTICS:
A total of 605 throat swabs were collected from outpatient children with community-acquired infections at the First Hospital of Hohhot between January 2022 and March 2023. During this period, China strictly enforced the use of medical masks, and intercity travel required negative nucleic acid testing. In Hohhot, China, in January 2022, schools closed, and entry into public places necessitated proof of a negative nucleic acid test. From October to December 2022, city residents faced restricted outdoor movements, with essential goods being delivered, and most public places were closed. Pathogen microarray analysis was performed on all patients, identifying various respiratory-related infections, and all pathogens detected in this study were transmitted through the respiratory tract but did not necessarily cause respiratory infections. The detected infections included: 262 cases of SARS-CoV-2, 56 cases of IAV, 83 cases of H. influenzae, 138 cases of S. pneumoniae, 43 cases of EBV, 26 cases of CMV, 52 cases of RSV, 42 cases of PIV, 25 cases of IBV, 8 cases of M. pneumoniae, 7 cases of ADV, and 33 cases of other respiratory pathogens (Table 1). These infections included both single and mixed infections. Among the patients, 56.03% were male, with an average age of 3.45 years. Infants aged 0–1 year accounted for 24.30%, children aged 1–3 years accounted for 40.50%, and those aged 3–16 years accounted for 35.21%. SARS-CoV-2 infections were distributed across all age groups, while RSV and CMV infections were more common in children under 3 years old. IAV, H. influenzae, S. pneumoniae, EBV, PIV, IBV, and M. pneumoniae infections were mainly observed in children above age 1 year.
PATHOGEN FREQUENCY AND SEASONALITY:
Figure 1 illustrates the frequency of different pathogen infections detected in patients during different months. More COVID-19 cases occurred in January 2022, and the hospital mainly focused on testing patients for COVID-19, neglecting the infection status of other patients. Stricter prevention and control measures were implemented in Hohhot in October and November 2022, during which only SARS-CoV-2 infections were tested for in hospitalized patients. Samples from other months were tested using pathogen microarray. SARS-CoV-2 cases persisted from October 2022 to January 2023 and then disappeared. IAV cases appeared in December 2022, with subtypes H1N1 (n=42), H3N2 (n=14), and H1N2 (n=3) identified. The number of IAV cases reached its peak in March 2023. S. pneumoniae and H. influenzae infections showed consistent occurrence, with more than 10 cases each month and no significant seasonality. EBV infections were mainly observed in summer 2022. RSV, IBV, PIV, M. pneumoniae, E. coli, S. aureus, GAS, and other pathogens were not detected between December 2022 and March 2023.
According to Tables 2 and 3, there were 365 patients of single infections, 174 patients with mixed infections, 113 patients with 2 pathogens, 61 patients with more than 2 pathogens, and 66 patients with no pathogens detected. Excluding the infection data from October to November 2022, mixed infections were more common for all types of pathogens except SARS-CoV-2. The proportion of mixed infections was 41.94% among SARS-CoV-2 patients, while other pathogens had mixed infection rates exceeding 57.14%. Among those co-infected with SARS-CoV-2, the highest co-infection rates were observed with IAV (n=15, 24.19%), S. pneumoniae (n=8, 12.90%), and CMV (n=7, 11.29%). Among those co-infected with IAV, the highest co-infection rates were observed with S. pneumoniae (n=18, 32.14%), SARS-CoV-2 (n=15, 26.79%), and H. influenzae (n=11, 19.64%).
The average age of CMV infection, significantly lower than 1 year old, was notably lower than for other infections. The mixed infection rate of CMV (single infection: mixed infection=3: 23) was significantly higher than for other pathogens. This suggests that CMV is more prevalent in infants with immature immune systems, potentially due to reduced immunity caused by other infections in these infants, leading to an increase in CMV nucleic acid-positive patients. Although EBV generally does not cause pneumonia, it can induce symptoms similar to those of URTIs. Among children with a single positive EBV detection, 7 out of 10 (70%) showed symptoms of fever and 7 out of 10 (70%) were diagnosed with URTIs. Additionally, EBV showed a higher rate of mixed infections, at 33 out of 43 (76%). The incidence rate of immunocompromised-related symptoms was 6 out of 43 for EBV and 3 out of 26 for CMV. The identification of atypical respiratory pathogens such as CMV and EBV in this study reflects their co-infection patterns with other respiratory pathogens.
SYMPTOMS AND CLINICAL DIAGNOSIS:
Among the recoded symptoms, listed from most common to least common, were fever (n=439, 72.56%), cough (n=321, 53.06%), convulsion (n=67, 11.07%), difficulty breathing (n=59, 9.75%), runny nose (n=31, 5.12%), vomiting (n=38, 6.28%), throat discomfort (n=56, 9.26%), diarrhea (n=28, 4.63%), hoarse voice (n=20, 3.31%), and dizziness (n=16, 2.64%) (Table 2). SARS-CoV-2 had the lowest proportion of coughing (n=104, 39.69%) among all pathogens, while SARS-CoV-2 (n=28,10.69%) and IAV (n=9,16.07%) were most commonly associated with throat discomfort. H. influenzae (n=14, 16.87%), IAV virus (n=11, 19.64%), and S. pneumoniae (n=29, 21.01%) were most frequently associated with convulsion. RSV (n=19, 36.54%), H. influenzae (n=13, 15.66%), and S. pneumoniae (n=18, 13.04%) were the pathogens most commonly associated with difficulty breathing.
Regarding clinical diagnoses of respiratory infections, approximately 30–40% of all patients were confirmed to have URTIs, with SARS-CoV-2 primarily manifesting as URTIs. RSV (n=51, 98.08%), PIV (n=33, 78.57%), and IBV (n=18,72.00%) had a higher proportion of confirmed of lobular pneumonia, while other pathogens were around 50% (Table 3). H. influenzae (n=6, 7.23%) and M. pneumoniae (n=4, 50.00%) had a higher proportion of confirmed lobar pneumonia. EBV (n=7, 16.28%), H. influenzae (n=12, 14.46%), IAV (n=11, 19.64%), S. pneumoniae (n=27, 19.57%), and IBV (n=2, 8.00%) had a higher proportion of confirmed of neurological diseases, while other pathogens were less than 10%. EBV (n=7, 16.28%) was most likely to cause digestive system diseases. S. pneumoniae (n=11, 7.97%) had the highest proportion of cardiovascular system symptoms. There were more patients with symptoms of immunocompromise caused by S. pneumoniae infection (n=15, 10.9%) and H. influenzae infection (n=7, 8.4%) than virus-infected patients. Mixed infections were associated with more severe outcomes for SARS-CoV-2, RSV, EBV, and ADV, while single infections were more severe for IAV virus. Patients with M. pneumoniae infections experienced more severe conditions.
IMPACT OF PREVENTION AND CONTROL MEASURES ON RESPIRATORY PATHOGEN INFECTIONS:
China gradually eased its SARS-CoV-2 control policies in December 2022. Based on the provided data, there was a significant change in the spectrum of respiratory pathogen infections among hospitalized children at the First Hospital in Hohhot. We further analyzed the changes in pathogen spectrum, clinical symptoms, diagnoses, and blood parameters before and after December 2022. From December 2022 to March 2023, patients infected with S. pneumoniae, H. influenzae, and EBV showed significant decreases in WBC (5.8×109/L vs 8.83×109/L), CRP (6.33 mg/L vs 18.36 mg/L), and PCT (0.24 ng/ml vs 1.11 ng/ml), approaching normal values compared to before December 2022 (Figure 2). Furthermore, the mean proportion of symptoms such as cough (67.72% vs 56.67%), difficulty breathing (15.19% vs 3.33%), running nose (7.59% vs 1.67%%) of EBV, S. pneumoniae, and H. influenzae were slightly higher before December 2022 compared to after that period (Figure 3). Similarly, the mean proportion of clinical diagnoses related to lobular pneumonia (67.09% vs 43.33%), digestive system diseases (13.29% vs 10.00%), lobar pneumonia (10.76% vs 6.67%), and other illnesses (17.09% vs 3.33%) of EBV, S. pneumoniae, and H. influenzae were higher before December 2022 compared to after that period (Figure 4). However, for SARS-CoV-2, the levels of CRP (8.77 mg/L vs 5.42 mg/L) and PCT (0.46 ng/ml vs 0.4 ng/ml) slightly increased after December 2022. The proportions of cough and lobular pneumonia associated with SARS-CoV-2 also showed a slight upward trend after December 2022.
Based on the above results, during the period of stricter SARS-CoV-2 control measures from January to November 2022, the symptoms and disease severity of patients infected with
Discussion
The SARS-CoV-2 pandemic has seen the successful implementation of strict control measures such as travel restrictions, social distancing, and improved hygiene practices, leading to a reduction in the overall frequency of respiratory infections caused by other pathogens [32,33]. In our study, these measures have effectively limited the transmission of various respiratory pathogens such as RSV, IBV, PIV,
The behavioral changes and preventive measures adopted during the SARS-CoV-2 pandemic, such as increased mask usage and hand hygiene practices, may have contributed to the reduced transmission of certain respiratory pathogens [42]. However, individuals who contract these pathogens may have limited prior exposure and immunity, making them more susceptible to severe illness [43,44]. Several studies have reported an increased incidence of respiratory pathogen infections following the relaxation of SARS-CoV-2 control measures. For example, the reopening of schools in Shanghai, China led to a significant rise in RSV and PIV cases [12]. Similarly, in the Chaoshan area of China, there was an upsurge in IBV,
There are several limitations to our study. Firstly, relying solely on microarray results for pathogen data without validation through alternative methods may have led to false positives or negatives due to residual or contaminating pathogenic nucleic acids. In fact, we identified 66 patients with no evidence of pathogen infection. Secondly, collecting pathogen detection samples exclusively from throat swabs may have hindered the detection of lower respiratory tract infections, and use of alternative sampling methods might have improved accuracy. Thirdly, differentiating between community-acquired and hospital-acquired infections in patients with chronic diseases presents a challenge that requires further investigation. Lastly, accurately assessing disease progression and severity is limited by measuring blood biochemical indicators only once, emphasizing the need for multiple measurements. Enhancements in microarray technology for improved pathogen detection accuracy and considering alternative tissue sampling sites are crucial. Additionally, an integrated approach combining symptoms, blood indicators, and pathogen results will enhance the treatment of respiratory infections.
Conclusions
The microarray analysis found various community-acquired infections in outpatient children: 262 SARS-CoV-2, 56 IAV, 83
Figures
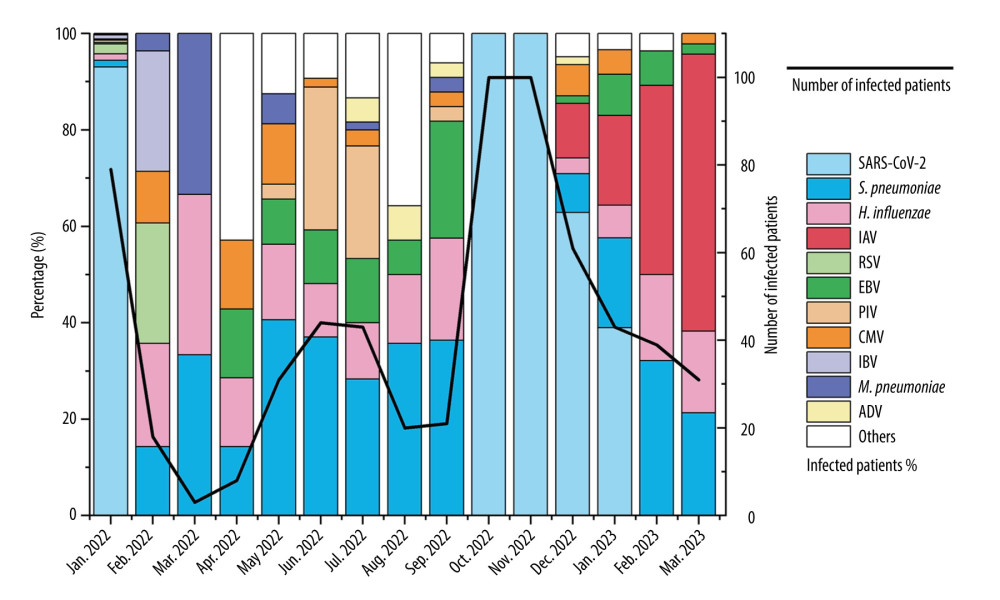 Figure 1. Monthly distribution of detected infections of respiratory pathogens in the First Hospital of Hohhot during the period January 2022 to March 2023. The “Others” category includes patients infected with MPV, GAS, E. coli, S. aureus, C. albicans, GBS, C. jejuni, Salmonella, K. pneumoniae, RV, and B. pertussis. There were fewer than 7 pathogens in infected patients.
Figure 1. Monthly distribution of detected infections of respiratory pathogens in the First Hospital of Hohhot during the period January 2022 to March 2023. The “Others” category includes patients infected with MPV, GAS, E. coli, S. aureus, C. albicans, GBS, C. jejuni, Salmonella, K. pneumoniae, RV, and B. pertussis. There were fewer than 7 pathogens in infected patients. 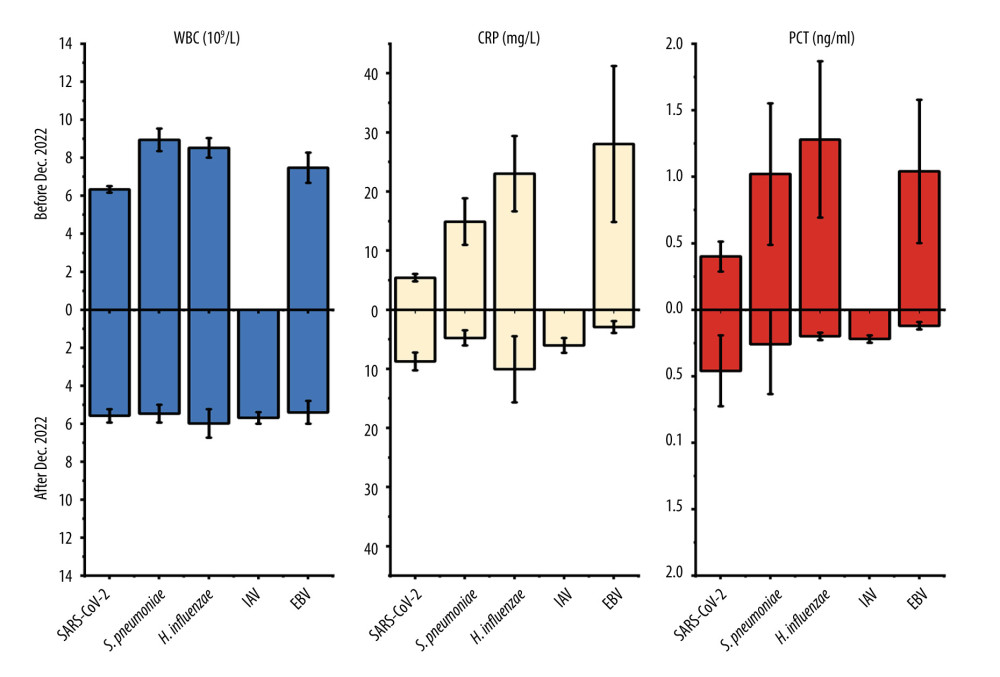 Figure 2. Values of WBC, CRP, and PCT in respiratory pathogen-infected patients detected before or after December 2022.
Figure 2. Values of WBC, CRP, and PCT in respiratory pathogen-infected patients detected before or after December 2022. 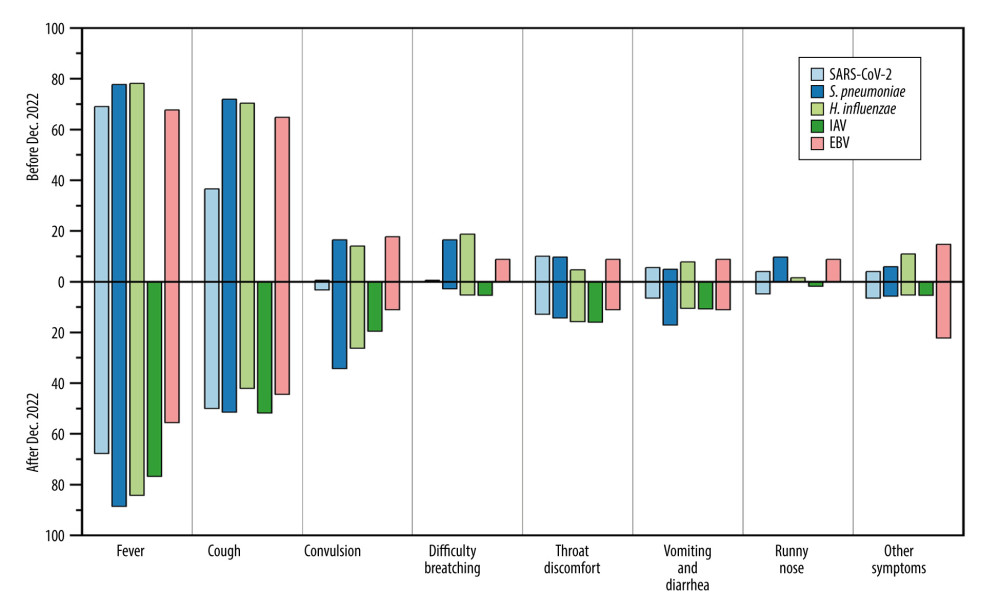 Figure 3. Mean proportion of symptoms in respiratory pathogen-infected patients detected before or after December 2022.
Figure 3. Mean proportion of symptoms in respiratory pathogen-infected patients detected before or after December 2022. 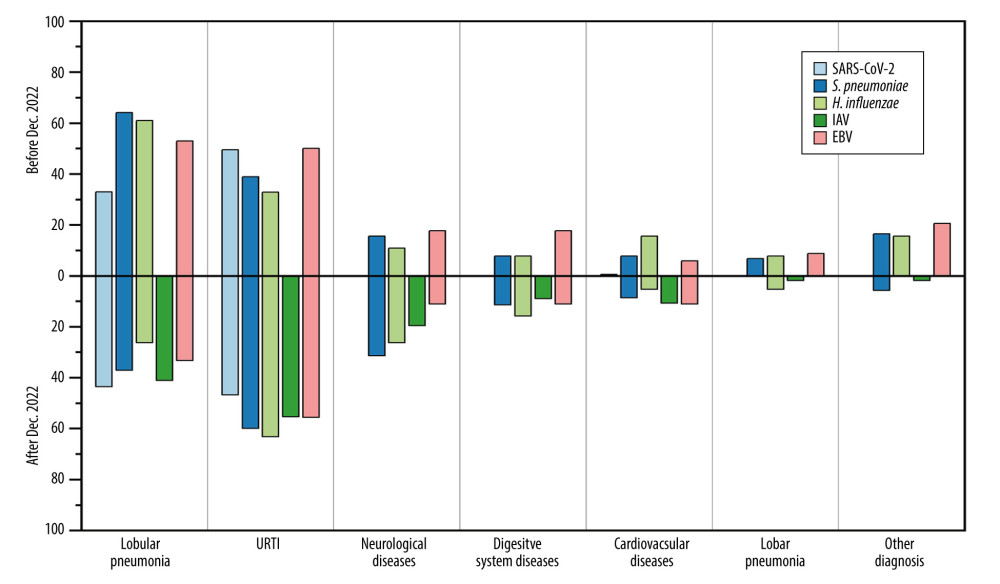 Figure 4. Mean proportion of clinical diagnoses in respiratory pathogen-infected patients detected before or after December 2022.
Figure 4. Mean proportion of clinical diagnoses in respiratory pathogen-infected patients detected before or after December 2022. Tables
Table 1. Baseline demographics and characteristics of respiratory pathogens in ARI patients (n=605).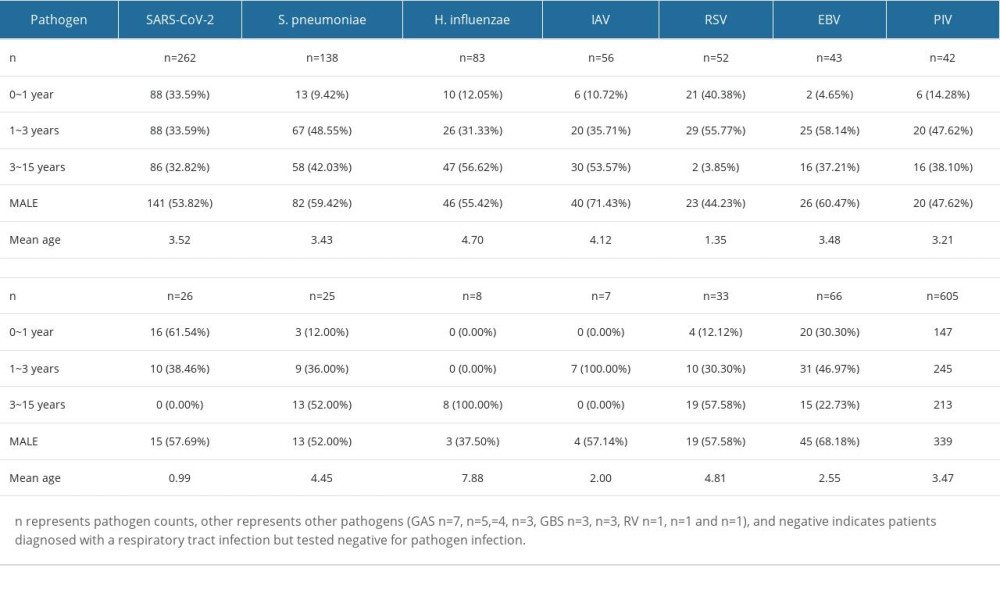 Table 2. Distribution characteristics of clinical symptoms and diseases caused by different respiratory pathogens in cases with single infection.
Table 2. Distribution characteristics of clinical symptoms and diseases caused by different respiratory pathogens in cases with single infection.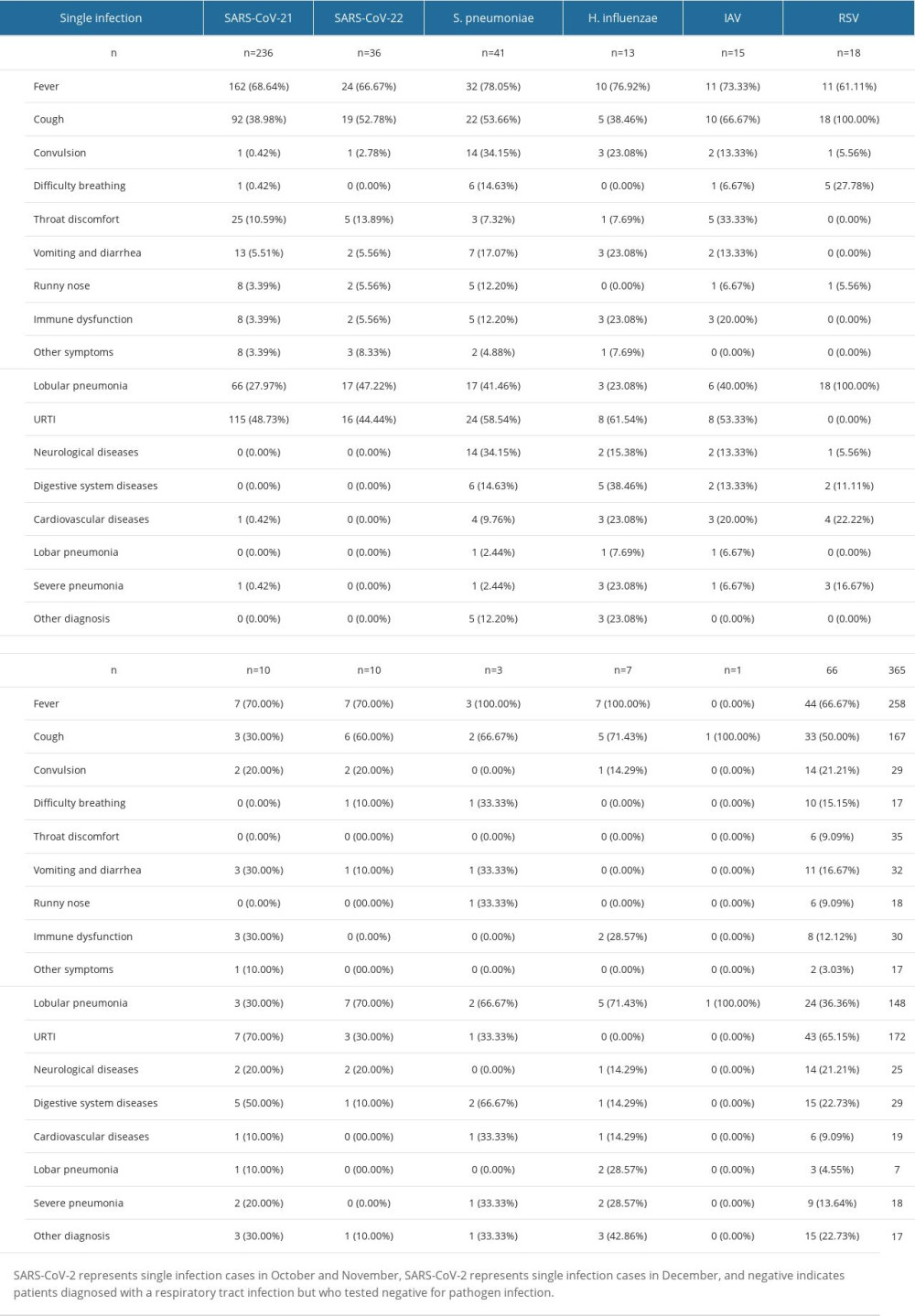 Table 3. Distribution characteristics of clinical symptoms and diseases caused by different respiratory pathogens in cases with mixed infection.
Table 3. Distribution characteristics of clinical symptoms and diseases caused by different respiratory pathogens in cases with mixed infection.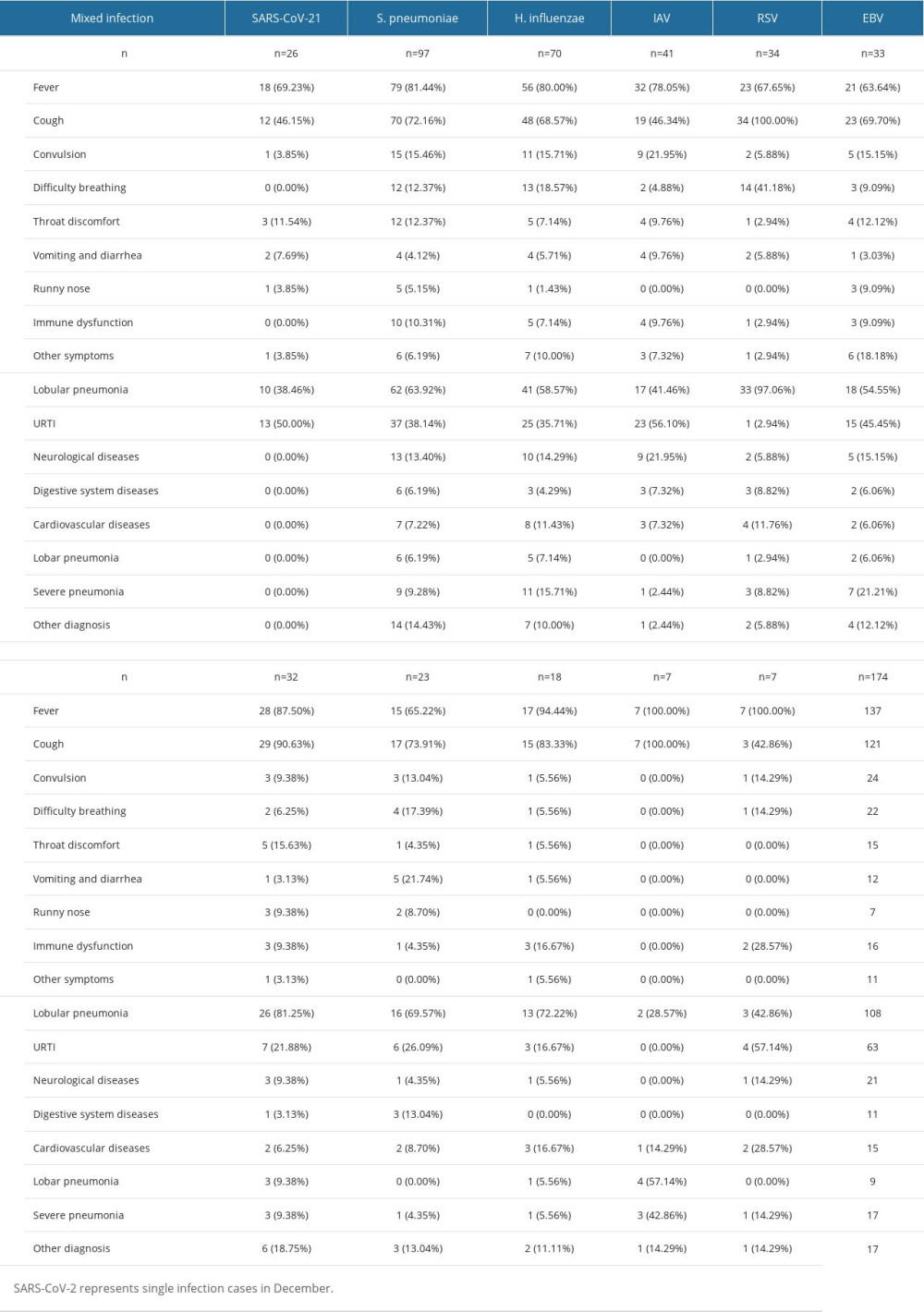
Reference
1. Cox NJ, Subbarao K, Influenza: Lancet, 1999; 354(9186); 1277-82
2. Kinder JT, Moncman CL, Barrett C, Respiratory syncytial virus and human metapneumovirus infections in three-dimensional human airway tissues expose an interesting dichotomy in viral replication, spread, and inhibition by neutralizing antibodies: J Virol, 2020; 94(20); e01068-20
3. Jain S, Self WH, Wunderink RG, Community-acquired pneumonia requiring hospitalization among U.S. adults: N Engl J Med, 2015; 373(5); 415-27
4. Musher DM, Thorner AR, Community-acquired pneumonia: N Engl J Med, 2014; 371(17); 1619-28
5. Benet T, Sanchez Picot V, Messaoudi M, Microorganisms associated with pneumonia in children <5 years of age in developing and emerging countries: The GABRIEL pneumonia multicenter, prospective, case-control study: Clin Infect Dis, 2017; 65(4); 604-12
6. Mina S, Yaakoub H, Annweiler C, COVID-19 and gungal infections: A double debacle: Microbes Infect, 2022; 24(8); 105039
7. Nair H, Nokes DJ, Gessner BD, Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis: Lancet, 2010; 375(9725); 1545-55
8. Morens DM, Fauci AS: J Infect Dis, 2007; 195(7); 1018-28
9. Jester BJ, Uyeki TM, Patel A, 100 years of medical countermeasures and pandemic influenza preparedness: Am J Public Health, 2018; 108(11); 1469-72
10. Johns Hopkins University School of Medicine Coronavirus-Resource Center Available from:https://coronavirusjhuedu/maphtml
11. Xinhuanet: China Focus: Schools start online courses as epidemic control postpones new semester, 2020 Available fromhttp://wwwxinhuanetcom/english/2020-02/17/c_138792006htm
12. Liu P, Xu M, Cao L, Impact of COVID-19 pandemic on the prevalence of respiratory viruses in children with lower respiratory tract infections in China: Virol J, 2021; 18(1); 159
13. den Hartog G, van Kasteren PB, Schepp RM, Decline of RSV-specific antibodies during the COVID-19 pandemic: Lancet Infect Dis, 2023; 23(1); 23-25
14. Jackson C, Vynnycky E, Hawker J, School closures and influenza: Systematic review of epidemiological studies: BMJ Open, 2013; 3(2); e002149
15. Markel H, Lipman HB, Navarro JA, Nonpharmaceutical interventions implemented by US cities during the 1918–1919 influenza pandemic: JAMA, 2007; 298(6); 644-54
16. Cowling BJ, Ali ST, Ng TWY, Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: An observational study: Lancet Public Health, 2020; 5(5); e279-e88
17. Olsen SJ, Azziz-Baumgartner E, Budd AP, Decreased influenza activity during the COVID-19 pandemic – United States, Australia, Chile, and South Africa, 2020: MMWR Morb Mortal Wkly Rep, 2020; 69(37); 1305-9
18. Zhang H, Lai X, Patenaude BN, Adding new childhood vaccines to China’s National Immunization Program: Evidence, benefits, and priorities: Lancet Public Health, 2023; 8(12); e1016-e24
19. Wu S, VANA L, Wang L, Estimated incidence and number of outpatient visits for seasonal influenza in 2015–2016 in Beijing, China: Epidemiol Infect, 2017; 145(16); 3334-44
20. Huang WJ, Cheng YH, Tan MJ, Epidemiological and virological surveillance of influenza viruses in China during 2020–2021: Infect Dis Poverty, 2022; 11(1); 74
21. Gan Y, Hu Y, Dong H, Causes of lower respiratory tract infections and the use of diagnostic biomarkers in blood samples from children in Hohhot, Inner Mongolia, China, between July 2019 and June 2020: Med Sci Monit, 2022; 28; e934889
22. Gan Y, Qi H, Li Y, Application of combined test of blood, C-reactive protein, and prealbumin in the differential diagnosis of pathogens for children’s upper respiratory tract infection: Int J Clin Exp Med, 2017; 10(1); 951-57
23. Galanti M, Shaman J, Direct observation of repeated infections with endemic coronaviruses: J Infect Dis, 2021; 223(3); 409-15
24. Guo L, Ren L, Yang S, Profiling early humoral response to diagnose novel coronavirus disease (COVID-19): Clin Infect Dis, 2020; 71(15); 778-85
25. Sreenath K, Batra P, Vinayaraj EV, Coinfections with other respiratory pathogens among patients with COVID-19: Microbiol Spectr, 2021; 9(1); e0016321
26. Hawke K, King D, van Driel ML, McGuire TM, Homeopathic medicinal products for preventing and treating acute respiratory tract infections in children: Cochrane Database Syst Rev, 2022; 12(12); CD005974
27. Gamero A, Belloch C, Ibanez C, Querol A: PLoS One, 2014; 9(5); e97626
28. Raabe VN, Shane AL: Microbiol Spectr, 2019; 7(2); 10.1128
29. Meena DS, Kumar D, Candida pneumonia: An innocent bystander or a silent killer?: Med Princ Pract, 2022; 31(1); 98-102
30. Cohen JI, Primary Immunodeficiencies associated with EBV disease: Curr Top Microbiol Immunol, 2015; 390(Pt 1); 241-65
31. Boeckh M, Ljungman P, How we treat cytomegalovirus in hematopoietic cell transplant recipients: Blood, 2009; 113(23); 5711-19
32. Marriott D, Beresford R, Mirdad F, Concomitant marked decline in prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and other respiratory viruses among symptomatic patients following public health interventions in Australia: Data from St Vincent’s Hospital and associated screening clinics, Sydney, NSW: Clin Infect Dis, 2021; 72(10); e649-e51
33. Hsieh CC, Lin CH, Wang WYC, The outcome and implications of public precautionary measures in Taiwan-declining respiratory disease cases in the COVID-19 pandemic: Int J Environ Res Public Health, 2020; 17(13); 4877
34. Feldman C, Anderson R, The role of co-infections and secondary infections in patients with COVID-19: Pneumonia (Nathan), 2021; 13(1); 5
35. Gertz AH, Pollack CC, Schultheiss MD, Brownstein JS, Delayed medical care and underlying health in the United States during the COVID-19 pandemic: A cross-sectional study: Prev Med Rep, 2022; 28; 101882
36. Bauchner H, Fontanarosa PB, Excess deaths and the great pandemic of 2020: JAMA, 2020; 324(15); 1504-5
37. Horwitz CA, Henle W, Henle G, Clinical and laboratory evaluation of infants and children with Epstein-Barr virus-induced infectious mononucleosis: Report of 32 patients (aged 10–48 months): Blood, 1981; 57(5); 933-38
38. Cherry JDHG, Kaplan SL, Steinbach W, Hotez P: Feigin and Cherry’s textbook of pediatric infectious diseases, 2019; 1429, Elsevier Saunders
39. Restrepo-Gualteros SM, Gutierrez MJ, Villamil-Osorio M, Challenges and clinical implications of the diagnosis of cytomegalovirus lung infection in children: Curr Infect Dis Rep, 2019; 21(7); 24
40. Kelly MS, Benjamin DK, Puopolo KM, Postnatal cytomegalovirus infection and the risk for bronchopulmonary dysplasia: JAMA Pediatr, 2015; 169(12); e153785
41. Lansbury L, Lim B, Baskaran V, Lim WS, Co-infections in people with COVID-19: A systematic review and meta-analysis: J Infect, 2020; 81(2); 266-75
42. Ayouni I, Maatoug J, Dhouib W, Effective public health measures to mitigate the spread of COVID-19: A systematic review: BMC Public Health, 2021; 21(1); 1015
43. Alaib H, Algariri N, Ahmed H, Frequency and seasonal variations of viruses causing respiratory tract infections in children pre- and post-COVID-19 pandemic in Riyadh (2017–2022): Cureus, 2023; 15(1); e33467
44. Baumkotter R, Yilmaz S, Zahn D, Protective behavior and SARS-CoV-2 infection risk in the population – results from the Gutenberg COVID-19 study: BMC Public Health, 2022; 22(1); 1993
45. Lin CX, Lian HB, Lin GY, Comparison of 14 respiratory pathogens among hospitalized children during and after the COVID-19 outbreak in Chaoshan area: Virol J, 2023; 20(1); 70
46. Sagnelli C, Macera M, Camaioni C, SARS-CoV-2 infection: A hurricane that does not ignore chronic hepatitis: Infection, 2022; 50(4); 849-58
47. Coleman JJ, Manavi K, Marson EJ, COVID-19: To be or not to be; that is the diagnostic question: Postgrad Med J, 2020; 96(1137); 392-98
Figures
 Figure 1. Monthly distribution of detected infections of respiratory pathogens in the First Hospital of Hohhot during the period January 2022 to March 2023. The “Others” category includes patients infected with MPV, GAS, E. coli, S. aureus, C. albicans, GBS, C. jejuni, Salmonella, K. pneumoniae, RV, and B. pertussis. There were fewer than 7 pathogens in infected patients.
Figure 1. Monthly distribution of detected infections of respiratory pathogens in the First Hospital of Hohhot during the period January 2022 to March 2023. The “Others” category includes patients infected with MPV, GAS, E. coli, S. aureus, C. albicans, GBS, C. jejuni, Salmonella, K. pneumoniae, RV, and B. pertussis. There were fewer than 7 pathogens in infected patients. Figure 2. Values of WBC, CRP, and PCT in respiratory pathogen-infected patients detected before or after December 2022.
Figure 2. Values of WBC, CRP, and PCT in respiratory pathogen-infected patients detected before or after December 2022. Figure 3. Mean proportion of symptoms in respiratory pathogen-infected patients detected before or after December 2022.
Figure 3. Mean proportion of symptoms in respiratory pathogen-infected patients detected before or after December 2022. Figure 4. Mean proportion of clinical diagnoses in respiratory pathogen-infected patients detected before or after December 2022.
Figure 4. Mean proportion of clinical diagnoses in respiratory pathogen-infected patients detected before or after December 2022. Tables
 Table 1. Baseline demographics and characteristics of respiratory pathogens in ARI patients (n=605).
Table 1. Baseline demographics and characteristics of respiratory pathogens in ARI patients (n=605). Table 2. Distribution characteristics of clinical symptoms and diseases caused by different respiratory pathogens in cases with single infection.
Table 2. Distribution characteristics of clinical symptoms and diseases caused by different respiratory pathogens in cases with single infection. Table 3. Distribution characteristics of clinical symptoms and diseases caused by different respiratory pathogens in cases with mixed infection.
Table 3. Distribution characteristics of clinical symptoms and diseases caused by different respiratory pathogens in cases with mixed infection. Table 1. Baseline demographics and characteristics of respiratory pathogens in ARI patients (n=605).
Table 1. Baseline demographics and characteristics of respiratory pathogens in ARI patients (n=605). Table 2. Distribution characteristics of clinical symptoms and diseases caused by different respiratory pathogens in cases with single infection.
Table 2. Distribution characteristics of clinical symptoms and diseases caused by different respiratory pathogens in cases with single infection. Table 3. Distribution characteristics of clinical symptoms and diseases caused by different respiratory pathogens in cases with mixed infection.
Table 3. Distribution characteristics of clinical symptoms and diseases caused by different respiratory pathogens in cases with mixed infection. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








