12 March 2024: Clinical Research
Maternal Vaccination in Pregnancy: An Assessment of Influenza, Pertussis, and COVID-19 Vaccination Rates in Cracow, Poland
Julia Jurga1ABCDEF*, Gabriela Mierzwa1ABDEF, Justyna Agnieszka Kuciel1ABDEF, Magdalena KołakDOI: 10.12659/MSM.943304
Med Sci Monit 2024; 30:e943304
Abstract
BACKGROUND: Maternal vaccination during pregnancy reduces the risk of severe course and complications from infections both for the mother and her child. As information regarding immunization status of pregnant women with recommended vaccines in Poland is scarce, this questionnaire-based study aimed to identify influenza, pertussis (whooping cough), and COVID-19 vaccination in 205 pregnant women in Cracow, Poland, between February and April 2023. Another objective was to assess whether any of the maternal factors might influence women’s decision to inoculate during pregnancy.
MATERIAL AND METHODS: An anonymous and self-reported questionnaire developed specifically for this study was disseminated among postpartum women, who gave birth and were hospitalized at the Department of Obstetrics and Perinatology of the University Hospital in Cracow, Poland, between February and April 2023. Study participants were asked about their basic sociodemographic and obstetric data, as well as their immunization status regarding influenza, pertussis, and COVID-19 during their most recent pregnancy.
RESULTS: Only 12.2% and 23.4% of study participants received influenza and pertussis vaccinations, respectively, during pregnancy, while 61.5% of pregnant women reported vaccination with at least 2 doses of the mRNA COVID-19 vaccine. Features including type of occupation, place of residence, gravidity, and parity were statistically significant (P<0.05) factors influencing the likelihood of maternal vaccination.
CONCLUSIONS: Influenza and pertussis vaccination coverage among pregnant women in Poland is suboptimal and lower than observed in most other countries, while COVID-19 immunization rates are on par with global trends.
Keywords: COVID-19 vaccine, Influenza Vaccines, Pertussis Vaccine, Pregnancy, Vaccination, Vaccination coverage
Background
Both pregnancy and early infancy represent stages of increased susceptibility to particular infections that can have a more severe course for individuals within these populations. As for expectant mothers, certain immunological (impaired cell-mediated immunity) and physiological changes (respiratory and cardiovascular alterations, such as decreased lung capacity and increased cardiac output) can result in a more complicated course of mainly viral infections, especially during the third trimester of pregnancy [1–3]. While the incidence of those infections, such as influenza, is comparable to that seen in the general population overall, hospitalization and mortality rates among pregnant women are reported to be higher, with a subsequently increased risk of adverse pregnancy and fetal outcomes [2–6]. As for neonates and infants, their immune system in the first months of life is still immature and incapable of active protection from infections, while administration of some vaccines is not yet possible [1,7]. Maternal vaccination during pregnancy can significantly reduce the risk of complications both for the mother and her child, before and after birth. This is mainly due to the mother’s capacity to produce IgG and IgA antibodies, which persist in women’s circulation and are transferred through the placenta or secreted with breastmilk [7,8].
Current Polish recommendations regarding vaccination during pregnancy are summarized in the recently published
The administration of the vaccines as mentioned above during pregnancy is reported to be safe, both for the mothers and their children, with an observed lower incidence of pertussis in neonates [12], as well as influenza [2], and COVID-19 [8,13,14] in both vaccinated mothers and their newborns.
Although recommendations to vaccinate pregnant women against influenza are a part of the Polish National Immunization Program, from 2014 [15], and against pertussis, from 2016 [4], information regarding the percentage of women receiving these vaccinations in Poland is scarce and not reported by any of the public institutions.
Therefore, this questionnaire-based study aimed to identify influenza, pertussis (whooping cough), and COVID-19 vaccination in 205 pregnant women in Cracow, Poland, between February and April 2023. Another objective was to assess whether any of the sociodemographic, clinical, and obstetric maternal features might influence women’s decision to inoculate during pregnancy.
Material and Methods
ETHICS STATEMENT:
Ethical approval was granted by the Bioethics Committee of the Jagiellonian University Medical College (opinion no. 1072.6120.16.2023). Due to the study’s anonymous design, the Bioethics Committee did not require written informed consent, as oral consent to take part in the study was considered sufficient. An appropriate statement regarding the lack of enclosing participant information form, informed consent, and personal data protection form was a part of the application submitted to and approved by the Bioethics Committee.
STUDY DESIGN:
An original survey was developed specifically for this study through a thorough literature review and a discussion in a group of research members, including obstetricians and specialists in maternal-fetal medicine, to appropriately assess pregnant women’s attitudes toward vaccination. The final questionnaire included a total of 28 single-choice, multiple-choice, and open-ended questions divided into 4 main sections. The first section included questions regarding basic sociodemographic data, such as age, level of education, occupation, place of residence, and comorbidities. In the second section, questions about obstetric data, such as gravidity, parity, time to conceive, and use of assisted reproductive technology, were included. As the third section of the survey focused on vaccination, study participants were asked whether they were vaccinated during pregnancy against influenza (Influvac Tetra or Vaxigrip Tetra), pertussis (Tdap), and COVID-19 (Pfizer-BioNTech or Moderna mRNA), about the overall number of mRNA COVID-19 vaccine doses administered, reasons to accept or decline vaccination during pregnancy, and sources of knowledge about vaccination. The fourth section of the questionnaire focused on prevention, understood as attending gynecologist visits. Due to the extensiveness of the questionnaire, in this article, we have focused mainly on the study participants’ basic sociodemographic and obstetric data, as well as their immunization status regarding influenza, whooping cough, and COVID-19 during pregnancy. The transcript of the entire questionnaire is shown in Table 1.
The study took place between February and April 2023 at the Department of Obstetrics and Perinatology of the University Hospital in Cracow, Poland, a tertiary healthcare center. Women who gave birth and were hospitalized at the Department were approached by the members of the scientific group with information describing the aims and objectives of the study, as well as the contents of the survey. If they expressed willingness and oral consent to take part in the study, they were given a questionnaire to be self-completed. For the survey to be anonymous, neither names nor surnames of the study participants were collected. Members of the research group collected filled questionnaires directly from the study participants to be stored in the Department’s safety vault, which could be accessed only by the scientific group members, to ensure the confidentiality of the responses. Once the study ended, answers from all the questionnaires were entered into a spreadsheet, with access restricted to research group members only, to facilitate statistical analysis. Because the data analysis took place after the completion of the whole study and given that the questionnaires were anonymous, members of the research group were not able to identify who was the respondent of a given survey.
INCLUSION AND EXCLUSION CRITERIA:
Women who gave birth and were hospitalized at the Department of Obstetrics and Perinatology of the University Hospital in Cracow, Poland, between February and April 2023 were eligible for inclusion. The study focused on women who were residents of Poland, while non-residents were not included in the survey, to maintain the demographic specificity of the study. Women who started but did not complete the survey were also excluded from the final analysis, to ensure data integrity and accuracy.
STATISTICAL ANALYSIS:
Statistical analysis was performed using IBM SPSS version 29. The distribution of descriptive variables was explored with the Shapiro-Wilk test. Variables with a normal distribution were presented as mean and standard deviation (SD) and those with a non-normal distribution as median and first and third quartile (Q1–Q3). The
Results
STUDY GROUP CHARACTERISTICS:
The questionnaire was completed by 205 out of 596 eligible women, resulting in a response rate of 34.4%. The mean age of the study group was 32.7 years. Most women declared being white-collar workers (72.6%), having a higher educational level (74.1%), living in cities with over 100 000 inhabitants (51.2%), and having more than 1 child (55.6%). Detailed sociodemographic, clinical, and obstetric features of the study participants are provided in Table 2.
VACCINATION DURING PREGNANCY:
During their most recent pregnancy, only 25 (12.2%) women were vaccinated against influenza, 48 (23.4%) against pertussis (whooping cough), and 126 (61.5%) were immunized with at least 2 doses of the mRNA COVID-19 vaccine (Table 3). Only 19 women (9.3%) had been fully inoculated with recommended vaccines per the Polish Guidelines [4].
COVID-19 VACCINATION:
Out of 17 women (8.3%) who received a mRNA COVID-19 vaccine during pregnancy, 1 of them (5.9%) received the second dose of the vaccine, 3 (17.6%) received the first booster shot (the third dose), and the remaining 13 (76.5%) received the second booster shot (the fourth dose). Detailed COVID-19 immunization status of the study group is provided in Table 4.
PREDICTORS OF VACCINE UPTAKE DURING PREGNANCY:
In the case of influenza, type of occupation (being a white-collar worker;
Discussion
To the best of our knowledge, this is the first Polish publication regarding both influenza and pertussis vaccination coverage among pregnant Polish women, in addition to assessing the COVID-19 immunization status within this cohort.
We found that only 12.2% and 23.4% of surveyed women were vaccinated during pregnancy against influenza and pertussis, respectively. However, 61.5% of study participants stated immunization with at least 2 doses of the mRNA COVID-19 vaccine. Only 8.3% of women received the mRNA COVID-19 vaccine during pregnancy, and it was mostly the second booster shot. As for predictors of vaccine uptake, maternal factors such as type of occupation, place of residence, gravidity, and parity influenced women’s decision to inoculate during pregnancy.
The influenza vaccination rate of 12.2% seems to be comparable to that seen in another group with a recommendation to vaccinate people in Poland ≥65 years old (around 10%), and higher than observed in the Polish population overall (about 3.5%) [16]. As for the findings from other Polish questionnaire-based studies, Pisula et al [17] estimated influenza vaccination coverage among pregnant Polish women in 2021 to be around 21% (with another 17.5% still considering vaccination during later stages of pregnancy), but in another publication by Jagielska et al [15] the willingness to vaccinate within this cohort during the 2016/2017 season was assessed to be as low as 3.5% (with even 68.0% vehemently opposed to inoculation). According to the latest European Centre for Disease Prevention and Control (ECDC) report [18], only 4 European countries (Hungary, Lithuania, Slovenia, Spain) officially reported influenza vaccination coverage rates among pregnant women during the 2018–2021 period, even though most of them (including Poland) have similar recommendations regarding influenza vaccination during pregnancy. The most recent immunization rates reported by the ECDC from the 2020/2021 period varied from as low as 1.7% to as high as 61.9% in Spain, far from reaching a European Union target of 75% in all high-risk groups of developing a severe course of infection [18]. As for the most up-to-date worldwide data, the influenza immunization rate among pregnant women in the United Kingdom was 35.0% during the 2022/2023 season [19], 47.2% in the United States for the 2022/2023 season [20], 53% in Canada in 2021 [21], and generally below 60% in Australia, ranging from 32 to 75% for the 2016–2021 period [22]. Comparing those estimates with the percentage of vaccinated women in our study, it seems that influenza vaccination coverage among the Polish pregnant cohort is much lower than observed in other high-income countries.
Pertussis vaccine uptake of 23.4% was almost twice as high as that of influenza (12.2%), possibly due to the expectant mothers’ different perceptions of those vaccinations. As previously stated in the literature, pregnant women may be more willing to receive the whooping cough vaccine, associated with infant protection, rather than influenza inoculation, which is perceived to mainly protect mothers [23]. Even though the cost of the pertussis vaccine in Poland has to be covered by the women themselves, whereas the influenza vaccine is free of charge [4], it seems that for Polish expectant mothers, it is essential to safeguard their yet unborn children from infection. Although there are some slight differences between countries about when precisely the vaccination should take place (eg, in the United Kingdom, it is recommended to vaccinate between week 16 and 32 of gestation) [12], most countries implemented similar guidelines as to those effective in Poland [24]. Even so, the pertussis vaccination coverage among pregnant Polish women assessed in our study seems to be visibly lower than that observed in the United Kingdom (60.7% during 2022/2023 period) [25], the United States (55.4% for 2022/2023 period) [20], Canada (65% in 2021) [21], and Australia (ranging from 49% to 89%, but predominantly greater than 70% during 2016–2021 period) [22].
As for the COVID-19 vaccination, 61.5% of surveyed women stated receiving at least 2 doses of the mRNA vaccine, with 34.6% immunized with at least 1 booster shot. These estimates are comparable to the vaccination coverage rates officially reported by the World Health Organization both in Poland (60.3% and 33.3%) and globally (66.3% and 31.9%) [26]. The most up-to-date US data reports 58.7% of pregnant women immunized with a primary dose schedule, and 27.3% receiving a booster shot (73.3% before pregnancy and 24.7% during its course) [20]. As for data regarding Polish expectant mothers, in a questionnaire-based study conducted by Nowacka et al [27] between 2021 and 2022 (approximately a year before our survey took place) 74% of surveyed women reported having COVID-19 vaccination, with 16% inoculating before pregnancy and 58% during its course (40% with primary dose schedule and 18% with booster shot). In our study, only 8.3% of women received the COVID-19 vaccine during pregnancy, mostly the second booster shot, as it was the time in Poland when the referrals to obtain the fourth dose of the vaccine were issued. This clearly shows that throughout the COVID-19 pandemic, the percentage of Polish women vaccinated with the primary dose schedule before rather than during pregnancy has gradually increased. Nowadays, expectant mothers provide themselves and their children additional protection against SARS-CoV-2 infection by receiving booster shots, which aligns with the current international recommendations. In the United Kingdom [11], all pregnant women should be offered the booster dose of the COVID-19 vaccine before the start of the third trimester, and both in Europe [28] and the United States [10], expectant mothers are advised to receive an updated 2023–2024 booster before the rise of SARS-CoV-2 infections during the winter season.
As for the sociodemographic, clinical, and obstetric predictors of vaccine uptake during pregnancy, type of occupation (being a white-collar worker) was a factor associated with inoculation with all 3 recommended vaccines. Although profession type seems to be inseparably related to the person’s educational background, this feature was not shown to be a statistically significant factor associated with vaccination, as it was in other publications [29,30]. In the case of influenza and COVID-19, living in big cities was a predictor of vaccine uptake, whereas in the literature, both living in urban [29,31] and rural [32] areas was related to inoculation during pregnancy. As for the pertussis vaccine, higher gravidity and parity have been previously associated with lower vaccination coverage [33]. It comes as no surprise as many women are not aware of the need to repeat inoculation in every subsequent pregnancy due to the waning of post-vaccination immunity after administration of the acellular booster [12]. Moreover, we found no difference between vaccinated and unvaccinated women in relation to their age, while prior publications have reported on both younger and older age being a statistically significant factor of vaccine uptake during pregnancy [29–32]. Similarly, no differences in comorbidities, time to conceive, and use of assisted reproductive technology were noted, although it would seem plausible that women with chronic illnesses or encountering problems with getting pregnant would be more cautious about their health, which could result in higher vaccination coverage among this cohort.
There are certain limitations to our study, with the most important one being a small sample size of 205 study participants. We also observed a rather low response rate of 34.4%, which may be a result of a relatively high percentage of women refusing to take part in our study, as they stated they had not been vaccinated during pregnancy, so they did not want to complete a questionnaire regarding this topic. Another limitation of our research was its self-reported and anonymous design, due to which it was not possible to verify the factual immunization status of the surveyed women. What is more, given the study’s setting in a tertiary healthcare center in one of Poland’s major cities, the percentage of highly educated women in our study group was possibly higher than is observed in the Polish population overall. Considering all of those factors, this study should be considered preliminary, and further research on a much bigger group of women coming from different social and healthcare backgrounds should be conducted. Nevertheless, this study demonstrated that there is a significant gap in implementing public health policy regarding maternal healthcare in Poland. As data regarding vaccine uptake among pregnant individuals in Poland is still scarce and not officially reported, our results can serve as a valuable point of reference for future publications. As we have analyzed only a fraction of our survey’s results, another publication regarding motivations to accept or decline vaccination during pregnancy and the crucial role of a gynecologist-obstetrician in endorsing vaccine uptake is to be expected.
Conclusions
The study finds that the influenza and pertussis vaccination coverage rates among pregnant women in Poland are suboptimal and lower than observed in most other countries. In contrast, the COVID-19 immunization rates of the Polish pregnant cohort seem to be comparable to the rates observed globally.
Tables
Table 1. Transcript of the questionnaire used in the study. Table 2. Study group characteristics.
Table 2. Study group characteristics.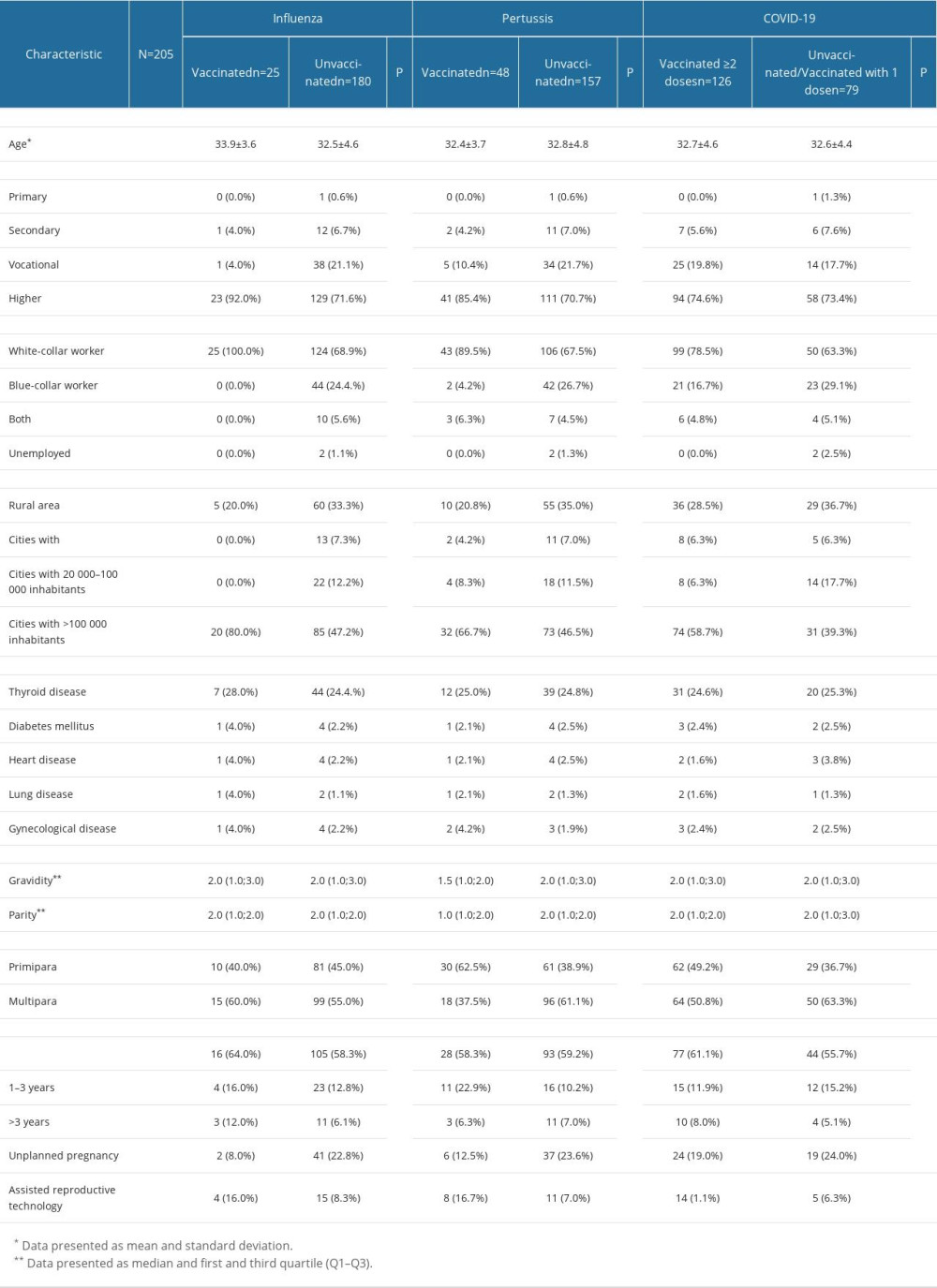 Table 3. The immunization status of the study participants regarding vaccines recommended during pregnancy (influenza, pertussis, and COVID-19), as per Polish Guidelines.
Table 3. The immunization status of the study participants regarding vaccines recommended during pregnancy (influenza, pertussis, and COVID-19), as per Polish Guidelines.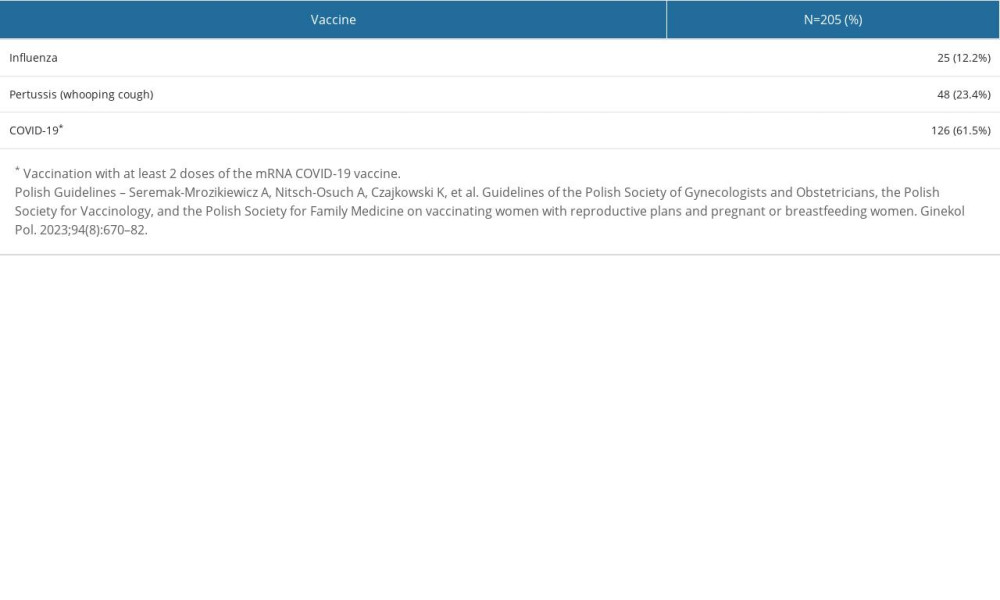 Table 4. COVID-19 vaccination status of the study group.
Table 4. COVID-19 vaccination status of the study group.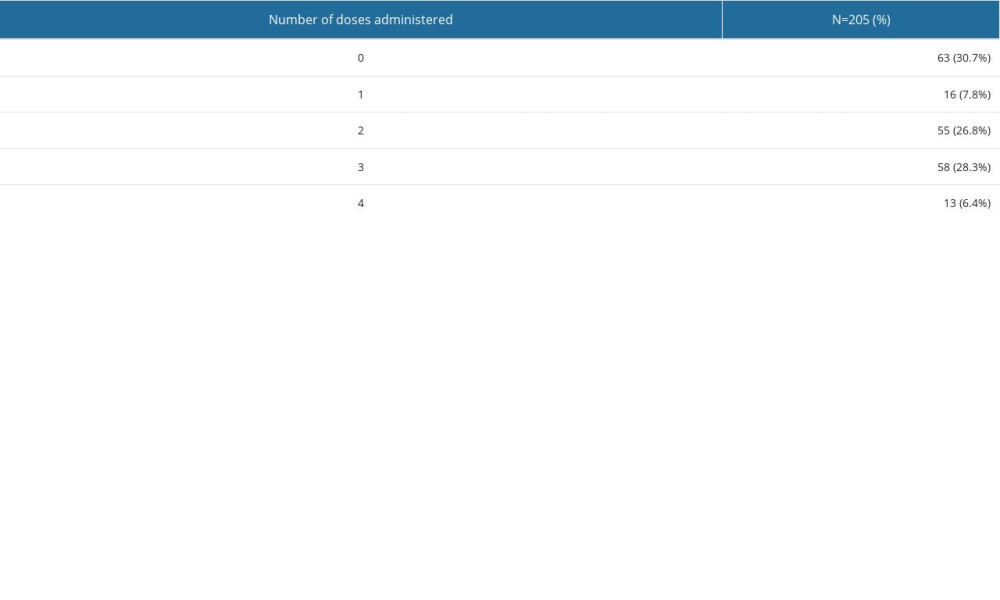
References
1. Etti M, Calvert A, Galiza E, Maternal vaccination: A review of current evidence and recommendations: Am J Obstet Gynecol, 2022; 226(4); 459-74
2. Nitsch-Osuch AS, Bomba-Opon D, Jasik M, Influenza vaccination in pregnancy – current data on safety and effectiveness: Ginekol Pol, 2020; 91(10); 629-33
3. Rasmussen SA, Jamieson DJ, Uyeki TM, Effects of influenza on pregnant women and infants: Am J Obstet Gynecol, 2012; 207(3); S3-S8
4. Seremak-Mrozikiewicz A, Nitsch-Osuch A, Czajkowski K, Guidelines of the Polish Society of Gynecologists and Obstetricians, the Polish Society for Vaccinology, and the Polish Society for Family Medicine on vaccinating women with reproductive plans and pregnant or breastfeeding women: Ginekol Pol, 2023; 94(8); 670-82
5. Skalska-świstek M, Huras H, Jaworowski AP, COVID-19 infection complicated by disseminated intravascular coagulation during pregnancy – two cases report: Diagnostics, 2022; 12(3); 655
6. Skalska-Swistek M, Kolak M, Jaworowski AP, Swistek R, Micek A, Huras H, SARS-CoV-2 infection during pregnancy – single-center retrospective study: Ginekol Pol, 2023; 94(10); 831-38
7. Albrecht M, Arck PC, Vertically transferred immunity in neonates: Mothers, mechanisms and mediators: Front Immunol, 2020; 11 Available from:https://www.frontiersin.org/articles/10.3389/fimmu.2020.00555/full
8. Blaszczyk E, Safety and efficiency of COVID-19 vaccination during pregnancy and breastfeeding: Ginekol Pol, 2022; 93(2); 168-72
9. , Committee Opinion No. 718: Update on immunization and pregnancy: Tetanus, diphtheria, and pertussis vaccination: Obstetr Gynecol, 2017; 130(3); e153-e57
10. American College of Obstetrics and Gynecology: COVID-19 vaccination considerations for obstetric-gynecologic care Available from [Published December 2020. Updated September 25, 2023https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care?utm_source=redirect&utm_medium=web&utm_campaign=int
11. Royal College of Obstetricians & Gynaecologists: Coronavirus (COVID-19) Infection in pregnancy Available from: [Published December 2022https://www.rcog.org.uk/media/ftzilsfj/2022-12-15-coronavirus-covid-19-infection-in-pregnancy-v16.pdf
12. Zasztowt-Sternicka M, Jagielska AM, Nitsch-Osuch AS, Pertussis vaccination in pregnancy – current data on safety and effectiveness: Ginekol Pol, 2021; 92(8); 591-94
13. Rahmati M, Yon DK, Lee SW, Effects of COVID-19 vaccination during pregnancy on SARS-CoV-2 infection and maternal and neonatal outcomes: A systematic review and meta-analysis: Rev Med Virol, 2023; 33(3); e2434
14. Zerbo O, Ray GT, Fireman B, Maternal SARS-CoV-2 vaccination and infant protection against SARS-CoV-2 during the first six months of life: Nat Commun, 2023; 14(1); 894
15. Jagielska AM, Jasik M, Nitsch-Osuch A, Determinants and coverage of seasonal influenza vaccination among women of childbearing age in Poland: Ginekol Pol, 2021; 92(1); 35-45
16. National Institute of Public Health NIH, National Research Institute Department of Epidemiology and Surveillance of Infectious Diseases: Chief Sanitary Inspectorate-Department of Epidemic Prevention and Border Sanitary Protection. Vaccinations in Poland in 2021 Available from:https://wwwold.pzh.gov.pl/oldpage/epimeld/2021/Sz_2021.pdf
17. Pisula A, Sienicka A, Pawlik KK, Dobrowolska-Redo A, Pregnant women’s knowledge of and attitudes towards influenza vaccination during the COVID-19 pandemic in Poland: Int J Environ Res Public Health, 2022; 19(8); 4504
18. European Center for Disease Control and Prevention, Seasonal influenza vaccination recommendations and coverage rates in EU/EEA Member States. An overview of vaccination recommendations for 2021-2022 and coverage rates for the 2018-19 to 2020-21 influenza seasons Available from: [Published October 9, 2023]https://www.ecdc.europa.eu/sites/default/files/documents/Seasonal-flu-vacc-recs-coverage-rates-EU-EEA.pdf
19. UK Health Security Agency: Seasonal influenza vaccine uptake in GP patients. Winter season 2022 to 2023 Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1177042/GP-patients-flu-annual-report-2022-2023.pdf
20. Razzaghi H, Kahn KE, Calhoun K, Influenza, Tdap, and COVID-19 vaccination coverage and hesitancy among pregnant women – United States, April 2023: MMWR Morb Mortal Wkly Rep, 2023; 72(39); 1065-71
21. Government of Canada: Results of the survey on vaccination during pregnancy 2021 Available from:https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/survey-vaccination-during-pregnancy-2021.html
22. McRae JE, McHugh L, King C, Influenza and pertussis vaccine coverage in pregnancy in Australia, 2016–2021: Med J Australia, 2023; 218(11); 528-41
23. Danchin MH, Costa-Pinto J, Attwell K, Vaccine decision-making begins in pregnancy: Correlation between vaccine concerns, intentions and maternal vaccination with subsequent childhood vaccine uptake: Vaccine, 2018; 36(44); 6473-79
24. Maltezou HC, Effraimidou E, Cassimos DC, Vaccination programs for pregnant women in Europe, 2021: Vaccine, 2021; 39(41); 6137-43
25. UK Health Security Agency: Prenatal pertussis vaccination coverage in England from January to March 2023 and annual coverage for 2022 to 2023 Available from: https://www.gov.uk/government/publications/pertussis-immunisation-in-pregnancy-vaccine-coverage-estimates-in-england-october-2013-to-march-2014/prenatal-pertussis-vaccination-coverage-in-england-from-january-to-march-2023-and-annual-coverage-for-2022-to-2023
26. World Health Organization: WHO Coronavirus (COVID-19) Dashboard Available from: https://covid19.who.int/table
27. Nowacka U, Malarkiewicz P, Sierdzinski J, COVID-19 vaccination status among pregnant and postpartum women – a cross-sectional study on more than 1000 individuals: Vaccines (Basel), 2022; 10(8); 1179
28. European Center for Disease Control and Prevention: ECDC-EMA statement on updating COVID-19 vaccines composition for new SARS-CoV-2 virus variants Available from:https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-vaccines-composition-variants-statement-ECDC-EMA_0.pdf
29. Lis-Kuberka J, Berghausen-Mazur M, Orczyk-Pawiłowicz M, Attitude and level of COVID-19 vaccination among women in reproductive age during the fourth pandemic wave: A cross-sectional study in Poland: Int J Environ Res Public Health, 2022; 19(11); 6872
30. Descamps A, Launay O, Bonnet C, Blondel B, Seasonal influenza vaccine uptake and vaccine refusal among pregnant women in France: Results from a national survey: Hum Vaccin Immunother, 2020; 16(5); 1093-100
31. Bienkowski C, Kowalczyk M, Golik A, The attitude of Polish women planning pregnancy and/or having children towards vaccinations: A cross-sectional survey study: Ginekol Pol, 2022; 93(8); 655-61
32. Okoli GN, Reddy VK, Al-Yousif Y, Sociodemographic and health-related determinants of seasonal influenza vaccination in pregnancy: A systematic review and meta-analysis of the evidence since 2000: Acta Obstet Gynecol Scand, 2021; 100(6); 997-1009
33. Razzaghi H, Kahn KE, Black CL, Influenza and Tdap vaccination coverage among pregnant women – United States, April 2020: MMWR Morb Mortal Wkly Rep, 2020; 69(39); 1391-97
Tables
 Table 1. Transcript of the questionnaire used in the study.
Table 1. Transcript of the questionnaire used in the study. Table 2. Study group characteristics.
Table 2. Study group characteristics. Table 3. The immunization status of the study participants regarding vaccines recommended during pregnancy (influenza, pertussis, and COVID-19), as per Polish Guidelines.
Table 3. The immunization status of the study participants regarding vaccines recommended during pregnancy (influenza, pertussis, and COVID-19), as per Polish Guidelines. Table 4. COVID-19 vaccination status of the study group.
Table 4. COVID-19 vaccination status of the study group. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








