03 May 2024: Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective Additive
Longlong Fu1ACEG*, Jing Ma2AFG, Lixia Chen3CF, Ying Guo4BCD, Wenjie Li5BCDE, Xingguo Zhang3BCD, Wenhong Lu1CDG, Shusong Wang2AFG, Ying Liu5ACDDOI: 10.12659/MSM.942946
Med Sci Monit 2024; 30:e942946
Abstract
BACKGROUND: Cryopreservation preserves male fertility, crucial in oncology, advanced age, and infertility. However, it damages sperm motility, membrane, and DNA. Zinc (Zn), an antioxidant, shows promise in improving sperm quality after thawing, highlighting its potential as a cryoprotectant in reproductive medicine.
MATERIAL AND METHODS: Gradient concentration of ZnSO₄ (0, 12.5, 25, 50, and 100 µM) was added in the Glycerol-egg yolk-citrate (GEYC) cryopreservative medium as an extender. Alterations in sperm viability and motility parameters after cryopreservation were detected in each group. Sperm plasma membrane integrity (PMI), acrosome integrity (ACR), DNA fragment index (DFI), and changes in sperm mitochondrial function were examined, including: mitochondrial potential (MMP), sperm reactive oxygen species (ROS), and sperm ATP.
RESULTS: We found that 50 µM ZnSO₄ was the most effective for the curvilinear velocity (VCL) and the average path velocity (VAP) of sperm after cryo-resuscitation. Compared to the Zn-free group, sperm plasma membrane integrity (PMI) was increased, DNA fragmentation index (DFI) was decreased, reactive oxygen species (ROS) was reduced, and mitochondrial membrane potential (MMP) was increased after cryorevival in the presence of 50 µM ZnSO₄.
CONCLUSIONS: Zn ion is one of the antioxidants in the cell. The results of our current clinical study are sufficient to demonstrate that Zn can improve preserves sperm quality during cryopreservation when added to GEYC. The addition of 50 µM ZnSO₄ increased curve velocity, mean path velocity, sperm survival (or plasma membrane integrity), and mitochondrial membrane potential while reducing ROS production and DNA breaks compared to GEYC thawed without ZnSO₄.
Keywords: Cryopreservation, Zinc, Human Sperm Motility, Mitochondrial Function
Introduction
Human sperm cryopreservation is currently the only effective clinical option for male fertility preservation in oncology patients, especially adolescents, and men in high-risk occupations and with advanced age [1]. Meanwhile, sperm cryopreservation can be combined with other assisted reproduction techniques in the treatment of patients with moderate or severe male infertility [2]. Sperm freezing has been available for decades. However, the freezing and thawing procedure leads to less motility, disrupted membranes, and more DNA fragments of sperm. Protecting male fertility, optimizing freeze-thaw technology, developing new cryoprotectants, and improving sperm quality after thawing are current research hotspots in reproductive medicine.
The damage of sperm from the freezing-thawing cycle includes the alteration of osmotic pressure, cold shock injury, formation of intracellular and extracellular ice crystals, toxicity of cryoprotectants, and oxidative stress [3]. Our previous work also showed that the damage from cryopreservation might result from genes/pathways associated with mitochondria, which are the major source of reactive oxygen species (ROS) [4,5].
A mixture of glycerol, egg yolk, and citrate (GEYC) cryopreservative medium has been widely used and is the recommended medium for human sperm preservation in the manual of the World Health Organization [6]. Oxidative stress plays an important role in cryoinjury, and the addition of different antioxidants to cryoprotectants can alleviate sperm cryoinjury to a certain extent and improve sperm quality after thawing [6,7].
Zinc (Zn), as one of the most abundant metals on earth, is an essential element for mammalian growth and development. With its moderate reactivity and reducing characteristics, Zn is a required cofactor for several metalloenzymes and acts as a catalyst for many enzymes [8]. Our team has been working on research related to Zn and male reproduction and has reported that Zn is involved in testicular growth, sperm quality and functional parameters, and male fertility [9,10]. In some animal studies, Zn can act as an antioxidant and improve sperm quality after cryopreservation [3,11]. Therefore, the aim of this study was to evaluate the effect of Zn, as a new cryoprotection additive, on human sperm quality during cryopreservation.
Material and Methods
ETHICS STATEMENT AND SAMPLE COLLECTION:
This study was conducted with the approval of the Human Subjects Ethics Committee of the National Research Institute for Family Planning (NRIFP2023024), and all the sperm donors provided written consent before participating in the study, agreeing to deliver their anonymous information for future studies.
All sperm samples were obtained from the Clinical Medical Center and Human Sperm Bank at the National Research Institute for Family Planning. The healthy volunteers met the following inclusion criteria: age between 25 and 35 years old, abstinence period of 5 to 7 days, semen volume of 4 to 6 mL, sperm concentration of 60 to 80×106/mL, sperm vitality >40%, and normal sperm morphology rate >4%, with no white cells or bacteria in the sperm. Volunteers with sexually transmitted diseases, cardiovascular diseases, endocrine diseases, or other conditions that could clearly affect sperm quality were excluded from the study.
Thirteen healthy volunteers were recruited, and semen samples, 3 mL each, were divided into 6 groups. The first group was untreated and was considered as the normal control group. The other 5 groups were frozen with GEYC medium and gradient concentrations of ZnSO4 (0, 12.5, 25, 50, and 100 μM) in liquid nitrogen for 2 weeks, and then thawed. Then, another 14 semen samples were screened to study the changes in the motility and functional parameters of sperm after cryopreservation with the addition of Zn ions.
In this study, a paired study design was used, with sperm parameters after cryo-resuscitation in the GEYC (without Zn) group as the experimental control group.
Unless stated otherwise, all the chemicals were procured from Merck.
SEMEN FREEZING AND THAWING PROTOCOLS:
The sperm freezing process was completely in accordance with the routine operation process performed in the sperm bank [4,5]. The cryoprotectant used was GEYC; 100 mL of GEYC contained 1.5 g of glucose, 1.3 g of sodium citrate, 1.3 g of glycine, 15 mL of glycerol, and 20 mL of fresh egg yolk. The semen sample was mixed with GEYC in a ratio of 2: 1 and was incubated at 30°C to 35°C for 5 min before being subjected to slow freezing using a programmable freezer. Then, the samples in tubes were cooled at 1.5°C per min from 20°C to −6°C, at 6°C per min to −100 °C, and at −100°C for 30 min. Then, the tubes were transferred to liquid nitrogen. After being preserved in the liquid nitrogen for a minimum of 2 months, the frozen samples were thawed, incubated at 37°C for 5 min, and underwent sperm quality assessment.
:
ZnSO4-7H2O was used as a cryoprotective additive. An amount of 28.756 g of ZnSO4-7H2O was dissolved in 100 mL of GEYC to obtain 1 M solution. Then 10 μL of the 1 M solution was added to 990 μL of GEYC to make a storage solution, with a Zn concentration of 1 mM. Then, the working solution of cryoprotectant was created, with Zn concentrations of 37.5, 75, 150, and 300 μM. The semen and GEYC were mixed in a 2: 1 ratio to make final solutions with Zn final concentrations of 12.5, 25, 50, and 100 μM.
ANALYSIS OF THE PARAMETERS OF SEMEN SAMPLES:
After the semen was fully liquefied, a Makler sperm counting chamber was used to calculate the concentration of sperm and sperm motility. Sperm motility parameters was evaluated by computer-aided sperm analysis (SuiPLus SSA-II, China). Thawed sperm motility was tested in the same way.
PLASMA MEMBRANE INTEGRIT:
Assessment of sperm plasma membrane integrity (PMI) was performed by eosin-aniline black staining. Breifly, 50 μL of semen and equal volume of eosin-aniline black suspension were mixed for 30 s (Huakang Company, China), and then 10 μL of the mixture was taken to make a smear. The smear was dried and observed under a bright field of view at 1000× magnification. The heads of spermatozoa with intact plasma membrane were stained white or light pink, and those with broken plasma membrane were stained red and dark pink. The percentage of sperm with intact plasma membrane was calculated and recorded as the PMI.
SPERM ACROSOME INTEGRITY:
Sperm acrosome integrity (ACR) was assessed using PSA-FITC staining. Approximately 2×104 spermatozoa were evenly spread on glass slides coated with 0.1% polylysine, air-dried, fixed in 95% (v/v) ethanol for 30 min, and then incubated with 3% PSA-FITC (3 μg/100 μL) for 1 h at 4°C in the dark. The slides were then rinsed 3 times with 0.1 M phosphate-buffered saline for 10 min each time. The slides were examined using a fluorescence microscope. Four hundred spermatozoa from each sample were analyzed. The slides were viewed with fluorescence optics at ×400 magnification with oil immersion at 450 nm to 490 nm excitation. The spermatozoa were categorized as (1) acrosome-intact: spermatozoa in which more than half the head is brightly and uniformly fluorescing; (2) acrosome-reacted: spermatozoa with only a fluorescing band at the equatorial segment or no fluorescing stain at all in the acrosome region; and (3) abnormal acrosomes: all other spermatozoa. The percentage of acrosome-intact and acrosome-reacted sperm was calculated and recorded as ACR.
SPERM DNA FRAGMENTATION INDEX:
The standardized test for chromatin structure analysis of spermatozoa was used to detect the DNA fragmentation index (DFI). Semen samples were diluted to a concentration of 1.0×106/mL with solution of 0.01 M Tris-HCl, 0.15 M NaCl, and 1 mM EDTA (pH 7.4). Denaturation of sperm DNA was induced with acidic washing buffer (0.1% Triton X-100, 0.15 M NaCl and 0.08 N HCl [pH 1.2]), followed by staining with acridine orange solution (pH 6.0). The fluorescence signals of 5000 to 10 000 spermatozoa were assessed by flow cytometry flow cytomete (Celula Medical Technology Co, China), and the DFI was calculated by dividing the number of red spermatozoa by the total number of red and green spermatozoa. The different colors of fluorescence reflected the integrity of and damage to sperm DNA, as evidenced by the red single-stranded and the green double-stranded breaks of DNA.
MITOCHONDRIAL MEMBRANE POTENTIAL:
The JC-1 method was used to assess the mitochondrial membrane potential (MMP) of spermatozoa. The sperm concentration was adjusted to 1×106/mL with Biggers, Whitten, and Whittingham (BWW) medium (95 mM NaCl, 4.6 mM KCl, 1.7 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5.6 mM glucose, 0.27 mM sodium pyruvate, 20 mM acid-free HEPES, 25 mM NaHCO3, 44 mM lactic acid, and 0.3% BSA [pH 7.4]), and the sperm suspension was added to the MMP assay kit (Solarbio, China) according to the following instructions. First, 0.5 mL of washed sperm was added to the MMP assay kit (Solarbio, China). The sperm concentration was adjusted to 1×106/mL with BWW medium, and the MMP assay kit (Solarbio, China) was used according to the manufacturer’s instructions. Then, 0.5 mL of JC-1 working solution was added to 0.5 mL of washed sperm suspension and incubated at 37 °C for 20 min. After incubation, spermatozoa were washed and resuspended with JC-1 staining buffer. The percentage of red or orange sperm/total sperm was calculated using flow cytomete (Celula Medical Technology Co, China) and recorded as MMP.
REACTIVE OXYGEN SPECIES:
Sperm reactive oxygen species (ROS) were assessed using the DCFH-DA method. Sperm were washed and resuspended as described above, using BWW medium. Sperm concentration was adjusted to 1×107/mL. Then 10 mmol/L DCFH-DA (Solarbio, China) was added at a ratio of 1: 1000 and incubated at 37°C for 20 min. After incubation, the spermatozoa were washed, and the concentration was readjusted for visualization. Staining effects and cellular localization were observed using fluorescence microscopy. Finally, the percentage of green spermatozoa/total spermatozoa was calculated by flow cytometry (Celula Medical Technology Co, China) and recorded as ROS.
ADENOSINE TRIPHOSPHATE:
The adenosine triphosphate (ATP) content in sperm was measured using an ATP assay kit (EZScreenTM ATP Colorimetric Assay Kit, Biovision, USA). Briefly, sperm samples (3×106 cells/mL) were homogenized with assay buffer (100 μL). Samples were treated with ATP reaction mixture in 96-well plates and gently shaken on a shaker for 2 min to induce sperm lysis. Samples were incubated in the dark for 30 min at room temperature. Finally, absorbance was measured at 570 nm using an enzyme marker (Tecan Infinite M200, Switzerland).
STATISTICAL ANALYSIS:
SPSS 22.0 and GraphpadPrism 6.0 were used for data statistics and graphing. The Kolmogorov-Smirnov test was used to test the normality of the data, and the data were normally distributed. Data with normal distribution were expressed as mean±standard deviation. Sperm parameters after cryo-resuscitation in the GEYC group (without Zn) were used as the experimental control group. The paired
Results
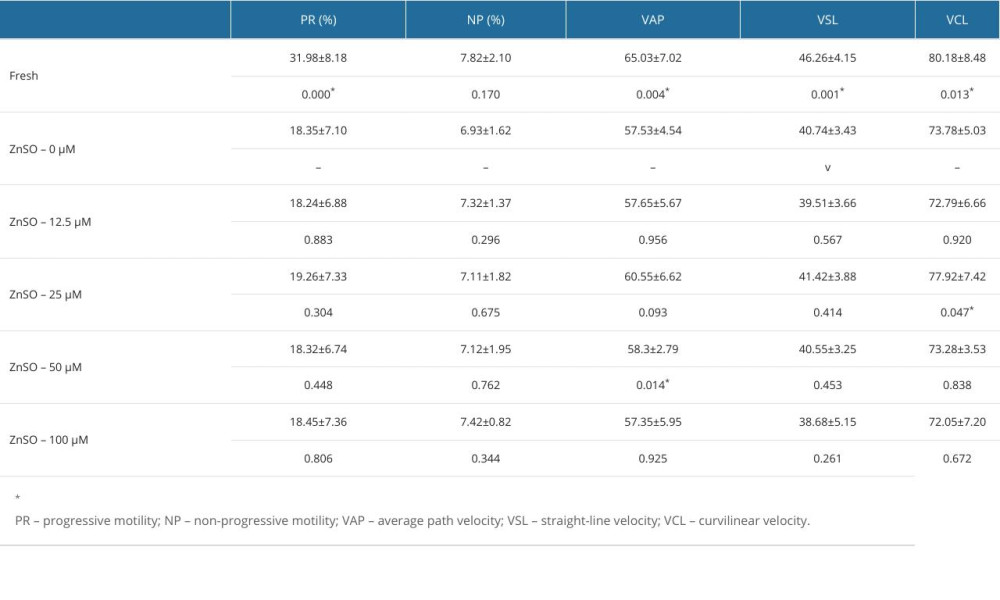
EFFECT OF ZN WITH DIFFERENT CONCENTRATIONS ON THE SPERM MOTILITY PARAMETERS (N=13):
The parameters of the semen samples were analyzed by computer-aided sperm analysis (Table 1). The curvilinear velocity (VCL, μm/s) of sperm with 25 μM ZnSO4 and the average path velocity (VAP, μm/s) of sperm with 50 μM ZnSO4 were significantly increased (P<0.05, Figure 1A, 1B). The P values were 0.047 and 0.014, respectively. Compared with the GYCE without ZnSO4, in the group of 50 μM ZnSO4, the VCL was increased by 6.72%, and the VAP was increased by 8.56%. However, the progressive motility, non-progressive motility, and straight-line (rectilinear) velocity (VSL, μm/s) were not altered significantly (P>0.05, Figure 1C–1E).
FUNCTION OF FROZEN-THAWED SPERM WITH AND WITHOUT ZN (N=14):
We found that not only the VAP was still significantly increased (P<0.01, Figure 2A), but also the VCL (P<0.05, Figure 2B). Significant improvements in PMI (P<0.001, Figure 2C), DFI (P<0.05, Figure 2D), ROS (P<0.01, Figure 2E), and MMP (P<0.01, Figure 2F) were observed in the 50 μM ZnSO4 group when compared with GYEC without Zn addition. However, the ACR and ATP were not significantly altered (P>0.05, Figure 3).
Discussion
STUDY LIMITATIONS:
As a clinical trial, we provide new directions for improving sperm viability and function after cryo-resuscitation. However, more clinical validation is needed at a later stage to better evaluate the safety and efficacy of Zn as a protective additive. On the basis of this study, we will conduct related metabolic and molecular studies to provide a stronger theoretical basis for the study of the mechanism of cryoinjury and the antioxidant effect of Zn to improve sperm function.
Conclusions
The Zn ion is one of the antioxidants in the cell. The results of our current clinical study are sufficient to demonstrate that Zn can improve preserved sperm quality during cryopreservation when added to GEYC. The addition of 50 μM ZnSO4 increased curve velocity, mean path velocity, sperm survival (or PMI), and MMP, while reducing ROS production and DNA breaks, compared with sperm in GEYC thawed without ZnSO4.
Figures
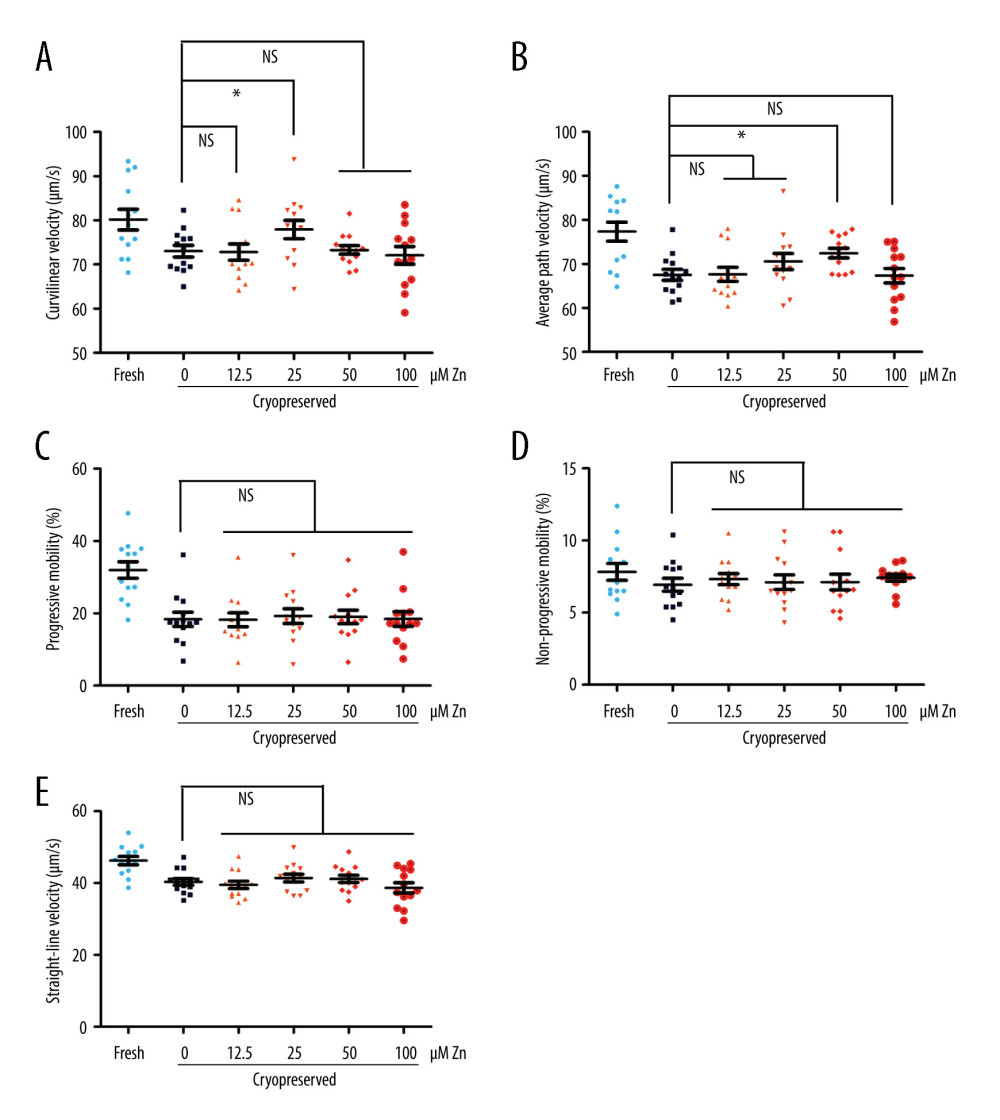 Figure 1. The parameters of semen cryopreserved with gradient concentration of ZnSO4. (A) The curvilinear velocity (VCL); (B) average path velocity (VAP); (C) progressive motility (PR); (D) non-progressive motility (NP); and (E) straight-line velocity (VSL) were examined. NS – no significant difference. * P<0.05. GraphPad Prism 5 (Dotmatics, San Diego, CA, USA).
Figure 1. The parameters of semen cryopreserved with gradient concentration of ZnSO4. (A) The curvilinear velocity (VCL); (B) average path velocity (VAP); (C) progressive motility (PR); (D) non-progressive motility (NP); and (E) straight-line velocity (VSL) were examined. NS – no significant difference. * P<0.05. GraphPad Prism 5 (Dotmatics, San Diego, CA, USA). 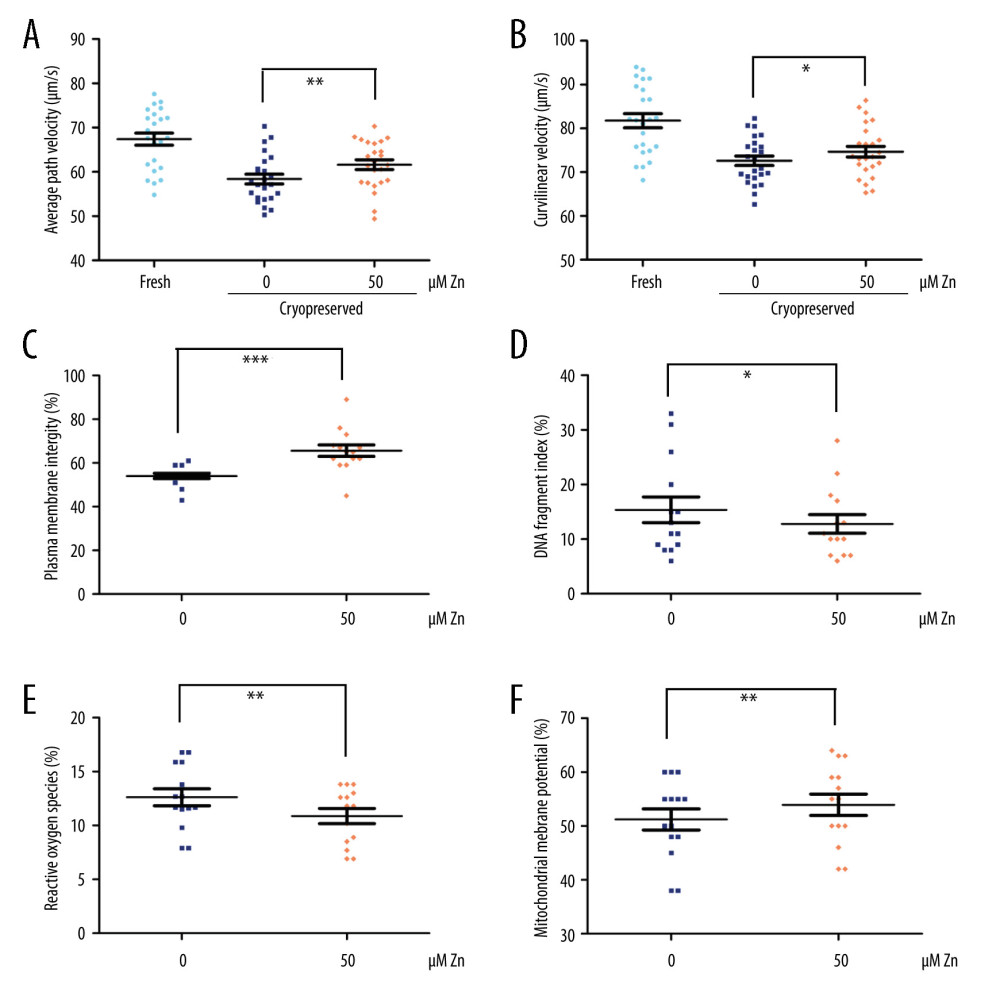 Figure 2. The parameters of semen cryopreserved with 50 μM ZnSO4. (A) The average path velocity (VAP); (B) curvilinear velocity (VCL); (C) sperm plasma membrane integrity (PMI); (D) DNA fragment index (DFI); (E) reactive oxygen species (ROS); and (F) mitochondrial membrane potential (MMP) were examined after cryopreservation with 50 μM ZnSO4. * P<0.05, ** P<0.01, *** P<0.001. GraphPad Prism 5 (Dotmatics, San Diego, CA, USA).
Figure 2. The parameters of semen cryopreserved with 50 μM ZnSO4. (A) The average path velocity (VAP); (B) curvilinear velocity (VCL); (C) sperm plasma membrane integrity (PMI); (D) DNA fragment index (DFI); (E) reactive oxygen species (ROS); and (F) mitochondrial membrane potential (MMP) were examined after cryopreservation with 50 μM ZnSO4. * P<0.05, ** P<0.01, *** P<0.001. GraphPad Prism 5 (Dotmatics, San Diego, CA, USA). 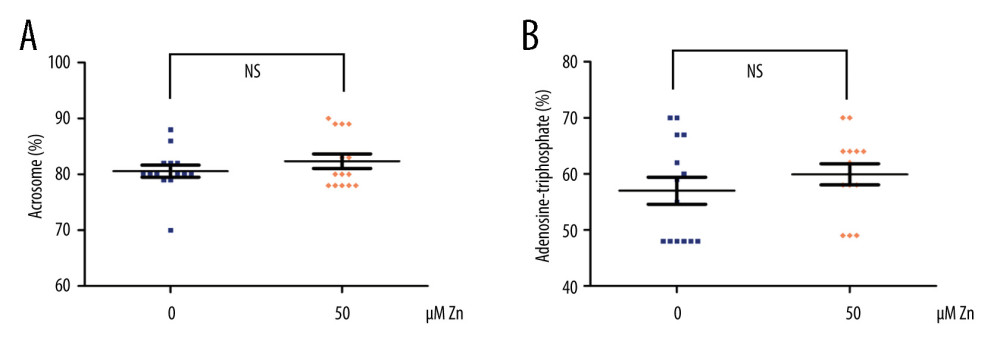 Figure 3. The parameters of semen cryopreserved with 50 μM ZnSO4. The percentage of (A) normal acrosome and (B) adenosine triphosphate (ATP) were examined. NS – no significant difference. GraphPad Prism 5 (Dotmatics, San Diego, CA, USA).
Figure 3. The parameters of semen cryopreserved with 50 μM ZnSO4. The percentage of (A) normal acrosome and (B) adenosine triphosphate (ATP) were examined. NS – no significant difference. GraphPad Prism 5 (Dotmatics, San Diego, CA, USA). References
1. Fu L, Zhou F, An Q, Sperm cryopreservation for male cancer patients: More than 10 years of experience, in Beijing China: Med Sci Monit, 2019; 25; 3256-61
2. Rienzi L, Gracia C, Maggiulli R, Oocyte, embryo and blastocyst cryopreservation in ART: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance: Hum Reprod Update, 2017; 23; 139-55
3. Ziarati N, Topraggaleh TR, Rahimizadeh P, Micro-quantity straw as a carrier for cryopreservation of oligozoospermic semen samples: Effects of storage times and cryoprotectant: Cryobiology, 2019; 86; 65-70
4. Fu L, An Q, Zhang K, Quantitative proteomic characterization of human sperm cryopreservation: Using data-independent acquisition mass spectrometry: BMC Urology, 2019; 19; 133
5. Fu L, Fang F, Guo Y, Combined analysis of the transcriptome, proteome and metabolome in human cryopreserved sperm: World J Mens Health, 2024 [Online ahead of print]
5a. World Health Organization: WHO Laboratory Manual for the Examination and Processing of Human Semen, 2021, Geneva, World Health Organization
6. Ozimic S, Ban-Frangez H, Stimpfel M, Sperm cryopreservation today: Approaches, efficiency, and pitfalls: Curr Issues Mol Biol, 2023; 45; 4716-34
7. Kerns K, Zigo M, Sutovsky P, Zinc: A necessary ion for mammalian sperm fertilization competency: Int J Mol Sci, 2018; 19(12); 4097
8. Lambert SA, Jolma A, Campitelli LF, The human transcription factors: Cell, 2018; 172; 650-65
9. Sun B, Ma J, Te L, Zinc-deficient diet causes imbalance in zinc homeostasis and impaired autophagy and impairs semen quality in mice: Biol Trace Elem Res, 2023; 201(5); 2396-406
10. Sun B, Ma J, Liu J, Mechanisms of damage to sperm structure in mice on the zinc-deficient diet: J Trace Elem Med Biol, 2023; 79; 127251
11. Yánez-Ortiz I, Catalán J, Rodríguez-Gil JE, Advances in sperm cryopreservation in farm animals: cattle, horse, pig and sheep: Anim Reprod Sci, 2022; 246; 106904
12. Arjun V, Kumar P, Dutt R, Effect of mitochondria-targeted antioxidant on the regulation of the mitochondrial function of sperm during cryopreservation: Andrologia, 2022; 54; e14431
13. Kotdawala AP, Kumar S, Salian SR, Addition of zinc to human ejaculate prior to cryopreservation prevents freeze-thaw-induced DNA damage and preserves sperm function: J Assist Reprod Genet, 2012; 29; 1447-53
14. Li Y, Lu T, Wu Z, Trends in sperm quality by computer-assisted sperm analysis of 49,189 men during 2015–2021 in a fertility center from China: Front Endocrinol (Lausanne), 2023; 14; 1194455
15. Escada-Rebelo S, Cristo MI, Ramalho-Santos J, Amaral S, Mitochondria-targeted compounds to assess and improve human sperm function: Antioxid Redox Signal, 2022; 37(7–9); 451-80
16. Gonzalez DC, Ory J, Blachman-Braun R, Advanced paternal age and sperm DNA fragmentation: A systematic review: World J Mens Health, 2022; 40(1); 104-15
17. Celino FT, Yamaguchi S, Miura C, Tolerance of spermatogonia to oxidative stress is due to high levels of Zn and Cu/Zn superoxide dismutase: PLoS One, 2011; 6(2); e16938
18. Xikeranmu Z, Abdunasir M, Ma J: Cryobiology, 2019; 87; 15-27
19. Nowicka-Bauer K, Lepczynski A, Ozgo M, Sperm mitochondrial dysfunction and oxidative stress as possible reasons for isolated asthenozoospermia: J Physiol Pharmacol, 2018; 69(3); 2018.3.05
20. Riffo M, Leiva S, Astudillo J, Effect of zinc on human sperm motility and the acrosome reaction: Int J Androl, 1992; 15(3); 229-37
21. Kerns K, Zigo M, Drobnis EZ, Zinc ion flux during mammalian sperm capacitation: Nat Commun, 2018; 9(1); 2061
22. Lee SR, Critical role of zinc as either an antioxidant or a prooxidant in cellular systems: Oxid Med Cell Longev, 2018; 2018; 9156285
Figures
 Figure 1. The parameters of semen cryopreserved with gradient concentration of ZnSO4. (A) The curvilinear velocity (VCL); (B) average path velocity (VAP); (C) progressive motility (PR); (D) non-progressive motility (NP); and (E) straight-line velocity (VSL) were examined. NS – no significant difference. * P<0.05. GraphPad Prism 5 (Dotmatics, San Diego, CA, USA).
Figure 1. The parameters of semen cryopreserved with gradient concentration of ZnSO4. (A) The curvilinear velocity (VCL); (B) average path velocity (VAP); (C) progressive motility (PR); (D) non-progressive motility (NP); and (E) straight-line velocity (VSL) were examined. NS – no significant difference. * P<0.05. GraphPad Prism 5 (Dotmatics, San Diego, CA, USA). Figure 2. The parameters of semen cryopreserved with 50 μM ZnSO4. (A) The average path velocity (VAP); (B) curvilinear velocity (VCL); (C) sperm plasma membrane integrity (PMI); (D) DNA fragment index (DFI); (E) reactive oxygen species (ROS); and (F) mitochondrial membrane potential (MMP) were examined after cryopreservation with 50 μM ZnSO4. * P<0.05, ** P<0.01, *** P<0.001. GraphPad Prism 5 (Dotmatics, San Diego, CA, USA).
Figure 2. The parameters of semen cryopreserved with 50 μM ZnSO4. (A) The average path velocity (VAP); (B) curvilinear velocity (VCL); (C) sperm plasma membrane integrity (PMI); (D) DNA fragment index (DFI); (E) reactive oxygen species (ROS); and (F) mitochondrial membrane potential (MMP) were examined after cryopreservation with 50 μM ZnSO4. * P<0.05, ** P<0.01, *** P<0.001. GraphPad Prism 5 (Dotmatics, San Diego, CA, USA). Figure 3. The parameters of semen cryopreserved with 50 μM ZnSO4. The percentage of (A) normal acrosome and (B) adenosine triphosphate (ATP) were examined. NS – no significant difference. GraphPad Prism 5 (Dotmatics, San Diego, CA, USA).
Figure 3. The parameters of semen cryopreserved with 50 μM ZnSO4. The percentage of (A) normal acrosome and (B) adenosine triphosphate (ATP) were examined. NS – no significant difference. GraphPad Prism 5 (Dotmatics, San Diego, CA, USA). In Press
20 Mar 2024 : Clinical Research
Prevalence and Management of Chronic Pain, Including Neuropathic Pain, in Dialysis Patients with End-Stage ...Med Sci Monit In Press; DOI: 10.12659/MSM.943808
15 Mar 2024 : Clinical Research
Impact of Cluster Nursing Intervention on ICU Patients' Psychological Well-Being and Complications Associat...Med Sci Monit In Press; DOI: 10.12659/MSM.942855
26 Mar 2024 : Clinical Research
New Computerized Planning Algorithm and Clinical Testing of Optimized Nuss Bar Design for Patients with Pec...Med Sci Monit In Press; DOI: 10.12659/MSM.943705
07 May 2024 : Clinical Research
Treatment of AVN-Induced Proximal Pole Scaphoid Nonunion Using a Fifth and Fourth Extensor Compartmental Ar...Med Sci Monit In Press; DOI: 10.12659/MSM.944553
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952