20 May 2024: Clinical Research
Diagnostic Efficiency of ACR-TIRADS Score for Differentiating Benign and Malignant Thyroid Nodules of Various Pathological Types
Yan Xu1ABCDEF, Jiashun Pi2CDEF, Yihan Jinghu2BCDEF, Xiaotao Wang1BCDE, Dong Xu3AEG, Jie Liu45ABCDEFG*DOI: 10.12659/MSM.943228
Med Sci Monit 2024; 30:e943228
Abstract
BACKGROUND: Thyroid nodule prevalence reaches 65% in the general population. Hence, appropriate ultrasonic examination is key in disease monitoring and management. We investigated the American College of Radiology Thyroid Imaging Reporting and Data System (ACR-TIRADS) score for diagnosis of benign and malignant thyroid nodules and pathological types.
MATERIAL AND METHODS: A retrospective study was conducted. According to ultrasound images, ultrasonic characteristics of benign and malignant thyroid nodules and different pathological types were analyzed using ACR-TIRADS score, and diagnostic value was determined. AUCs were compared for tumor diagnosis and differentiation.
RESULTS: Overall, 1675 thyroid nodules from 1614 patients were included. AUC value of papillary thyroid carcinoma (PTC) diagnosed with ACR-TIRADS was highest (0.955 [95% CI=0.946-0.965]), while that of follicular thyroid carcinoma (FTC) was lowest (0.877 [95% CI=0.843-0.912]). FTC had the highest sensitivity (95.1%) and lowest specificity (64.8%). When the cut-off value was 5.5 points, accuracy of diagnosing PTC and anaplastic thyroid carcinoma (ATC) was highest, 80.5% and 78.7% respectively. Comparison of the multi-index prediction model constructed by multivariable logistic regression analysis and prediction model constructed by ACR-TIRADS score showed, when evaluating PTC and ATC, the multi-index model was better: AUCs of PTC were 0.966 vs 0.955, and AUCs of ATC were 0.982 vs 0.952, respectively, (P<0.05).
CONCLUSIONS: ACR-TIRADS score-based ultrasound examination of thyroid nodules aids diagnosis of benign and malignant thyroid nodules. TIRADS criteria favor diagnosis of PTC (and ATC) over FTC. ACR-TIRADS score can help clinicians diagnose thyroid nodules quickly and earlier, exhibits good clinical value, and can prevent missed diagnoses.
Keywords: Imaging, Three-Dimensional, Thyroid Neoplasms, Ultrasonic Therapy
Introduction
Thyroid nodules have a prevalence of up to 65% in the general population [1,2]. The incidence of thyroid cancer has increased at rate of 3% per year, and the incidence of advanced papillary thyroid carcinoma (PTC) and the associated mortality has also increased [3]. This increase could be attributed to the widespread use of medical imaging and medical health surveillance, as well as to improvements in healthcare [4]. Thyroid ultrasound imaging can be used to identify patients at low risk for cancer [5–7] as well as those requiring fine-needle biopsy [8]. Fine-needle biopsy and molecular testing can reduce unnecessary testing and the related expenses to a great extent. However, the inappropriate use of fine-needle biopsy has the potential for increased medical risk, as does the cytological uncertainty that warrants surgery. Hence, appropriate ultrasonic examination is a key step in the effective monitoring and management of thyroid cancer [9,10].
The American College of Radiology Thyroid Imaging Reporting and Data System (ACR-TIRADS) assigns a score to thyroid nodules based on their ultrasonic features; the sum of the scores determines the nodule’s risk level and the need for biopsy or follow-up [11,12]. Hence, this system is highly effective and easily available [13–15]. Grani et al [15] compared 5 internationally recognized ultrasound classification systems, the American Thyroid Association (ATA), American Association of Clinical Endocrinologists (AACE), American College of Radiology (ACR), European Thyroid Association, and Korean Society of Thyroid Radiology systems, and found that ACR-TIRADS was most effective in ruling out malignant nodules and in differentiating benign and malignant nodules, thus reducing unnecessary fine-needle aspiration [16–18]. In another multicenter study, ACR-TIRADS was found to be more effective than the ATA guidelines and the Korean Society of Thyroid Imaging Reporting and Data System (KTIRADS) in reducing the number of unnecessary biopsies [19,20]. Therefore, ACR-TIRADS grading is a valuable tool for the diagnosis of thyroid nodules and is worthy of adoption in clinical practice [21].
Most current classification systems, including ACR-TIRADS, focus on PTC because of its high incidence. However, the annual incidence of medullary thyroid cancer (MTC) and follicular thyroid cancer (FTC) has also increased significantly [11,22], and the prognosis of different pathological types of thyroid cancer varies greatly [23]. Those with poor prognosis, such as anaplastic thyroid cancer (ATC), have almost no treatment options [24]. Many studies have used the ACR-TIRADS scoring system to evaluate benign and malignant thyroid nodules, but there are relatively few studies on the different pathological classifications of malignant thyroid nodules [25]. In this study, we analyzed and compared ultrasound images of benign and malignant thyroid nodules and different pathological classifications of malignant nodules based on the ACR-TIRADS scoring standard, evaluated its diagnostic performance for benign and malignant thyroid nodules, and evaluated the different pathologies of malignant nodules, which will in turn provide a solid basis for clinicians in diagnosis and treatment.
Material and Methods
PATIENT SELECTION:
A retrospective study was conducted in patients with thyroid nodules who underwent surgery in the hospital and had histopathology results from June 2009 to December 2022. To make a more in-depth study of different pathological types of patients, considering the low proportion of MTC, FTC, and undifferentiated carcinoma, this study included all patients with these cancer types in the past 10 years and included some patients with PTC. A total of 1614 patients were included in the study, including those with benign nodules (n=799) and malignant nodules (n=815), including PTC (n=631), MTC (n=85), FTC (n=80), and undifferentiated carcinoma (n=19). This retrospective study was approved by the Zhejiang Cancer Hospital, and the requirement for informed consent was waived by the Zhejiang Cancer Hospital and Zhejiang Rongjun Hospital and Shanghai University of Traditional Chinese Medicine.
Inclusion criteria were (1) patients who received preoperative ultrasonic examination in our hospital with complete ultrasound data and (2) patients who underwent surgery at our hospital with clear pathological findings.
Exclusion criteria were (1) patients for whom no ultrasonic examination data or images were available preoperatively; (2) the anatomical location of available images was not consistent with the description of pathological reports; and (3) there was a history of previous thyroid surgery.
ULTRASONIC EXAMINATION:
The Logiq E9 (GE Healthcare, USA), Toshiba 790A (Toshiba Corp, Japan), Esaote Mylab90 (Esaote S.P.A, Italy), and Philips iU22 (Philips, Netherlands) ultrasonic systems were used in this study. The probe types included ML6-15, PLT-805AT, LA523, and L12-5, and the probe frequency was 5–13 MHz.
The patient was in the supine position with neck hyperextension, and the transverse and longitudinal sections of the thyroid gland and adjacent tissues were carefully scanned. Information on the location, size, number, echogenicity, composition, morphology, margin, and calcification of the thyroid nodules was recorded. When evaluating nodules, each nodule feature was assigned a score according to the ACR-TIRADS guidelines criteria [13], as follows: (1) composition: cystic or almost completely cystic 0 points; spongy 0 points; mixed cystic and solid 1 point; solid or almost completely solid 2 points; (2) echo: no echo 0 points; high echo or equal echo 1 point; low echo 2 points; very low echo 3 points; (3) shape: wider than high 0 points, taller than wide, 3 points; (4) edge: smooth 0 points, unclear edges 0 points, lobulated or irregular 2 points, extrathyroidal extension 3 points; and (5) echo focus: no or large comet tail artifacts are scored as 0 points, coarse calcifications are scored as 1 point, peripheral calcifications are scored as 2 points, and punctate strong echoes are scored as 3 points.
Based on the ultrasound images of each thyroid nodule, the ACR-TIRADS score was used to analyze the sonographic characteristics of benign and malignant thyroid nodules and different pathological classifications [13]. Pairwise analysis and comparison between groups was performed on the 4 pathological types of malignant thyroid nodules according to the ACR-TIRADS scoring criteria. Finally, the diagnostic ability of the ACR-TIRADS score on benign and malignant thyroid nodules and different pathological classifications was determined through the area under the receiver operating characteristic (ROC) curve (AUC) and cut-off value.
DATA ANALYSIS:
SPSS (version 23) was used for statistical analyses. Descriptive variables are displayed as the mean±standard deviation values, and categorical variables are displayed as a percentage. The measurement data were compared by independent-sample
Results
PATIENT SCREENING:
In this study, 1675 thyroid nodules in 1614 patients (1235 women and 440 men; average age=49.1±12.91 years; age range=12–86 years) were involved. Among them, 846 thyroid nodules were benign, including 637 nodular goiters, 191 adenomatous hyperplasia nodules, and 18 adenomas. However, 829 thyroid nodules in 815 patients were malignant, including 631 PTC, 96 MTC, 81 FTC, and 21 ATC. The benign and malignant nodules varied significantly in terms of patient age, maximum diameter, location, composition, echogenicity, morphology, margin, calcification, ACR-TIRADS score, and classification (P<0.05). No differences were found by sex (P>0.05). The details are shown in Table 1.
ULTRASONIC FEATURES OF MALIGNANT THYROID NODULES OF DIFFERENT PATHOLOGICAL TYPES:
According to the ultrasonic features of malignant thyroid nodules of the 4 pathological types, taller-than-wide shape was commonly found in PTC (384, 60.9%). Mixed cystic and solid, and hyperechoic or isoechoic were common proportions in FTC (Figure 1), at 9.9% and 4.9%, respectively. Fine dot-like calcifications and extreme hypo-echogenicity were more common in MTC (Figure 2), at 39.6% (n=38) and 16.7% (n=16), respectively. ATC was characterized mainly by extracapsular thyroid invasion, in 47.6% (n=10), as detailed in Table 2.
COMPARISON OF MALIGNANT THYROID NODULES OF 4 PATHOLOGICAL TYPES BASED ON THE ACR-TIRADS CRITERIA:
Table 3 describes the distribution of ACR scores in 4 pathological types of thyroid malignant nodules. Based on the results of pairwise comparison with benign nodules at the controls between groups, we concluded that the ACR scores of FTC were significantly different from those of PTC, MTC, and thyroid failure (P<0.05). The difference in differentiated cancer (ATC) was statistically significant, P<0.05; the difference in ACR-TIRADS scores between PTC and MTC was also statistically significant (P<0.05; Figure 3, Table 4).
COMPARING DIAGNOSTIC ACCURACY OF ACR-TIRADS SCORE FOR BENIGN AND MALIGNANT THYROID NODULES AND MALIGNANT NODULES OF DIFFERENT PATHOLOGICAL TYPES BASED ON AUC:
As shown in Figure 4, the AUC of the ACR score for the diagnosis of malignant thyroid nodules was 0.942 (95% confidence interval [CI]: 0.932, 0.953). The AUC for the diagnosis of PTC was 0.955 (95% CI: 0.946, 0.965), the AUC for the diagnosis of MTC was 0.914 (95% CI: 0.886, 0.942), the AUC for the diagnosis of FTC was 0.877 (95% CI: 0.843, 0.912), and the AUC for the diagnosis of undifferentiated carcinoma was 0.952 (95% CI: 0.917, 0.988). For all thyroid nodules, the Youden index, cut-off, sensitivity of and specificity of ACR score in the diagnosis of thyroid malignant nodules and different pathological types are shown in Table 5.
LOGISTIC REGRESSION SCREENING VARIABLES AND CONSTRUCTING PREDICTION MODELS:
Through single-factor and multi-factor logistic regression analysis, it was determined that age, max_size, composition, echogenicity, margin, point, and TIRAD risk score (TR) were independent predictors of malignant thyroid nodules (Table 6). Composition, echogenicity, margin, echogenic_foci, point, and TR were independent predictors of PTC (Table 7). Composition, echogenicity, margin, shape, and TR were independent predictors of MTC (Table 8). Max_size, composition, echogenicity, margin, and TR were independent predictors of FTC (Table 9). Age, max_size, margin, and TR were independent predictors of ATC (Table 10). The predictors were jointly used to construct a prediction model and were combined with the prediction model constructed using the ACR-TIRADS score.
The AUC of ROC curve of the thyroid malignant nodule prediction model constructed by combining predictors was AUC of 0.961 (95% CI: 0.952–0.969), with sensitivity of 0.924, and specificity of 0.877. The AUC of the model constructed using the ACR-TIRADS score was 0.942 (95% CI: 0.932–0.952), with sensitivity of 0.929, and specificity of 0.826 (
The AUC of ROC curve of the PTC prediction model constructed by combining predictors was 0.966 (95% CI: 0.958–0.975), with sensitivity of 0.957, and specificity of 0.865. The AUC of the prediction model constructed using ACR-TIRADS scores was 0.955 (95% CI: 0.946–965), with sensitivity of 0.930, and specificity of 0.875 (
The AUC of ROC curve of the MTC prediction model constructed by combining predictors was 0.939 (95% CI 0.915–0.964), with sensitivity of 0.975, and specificity of 0.729. The AUC of the prediction model constructed using ACR-TIRADS scores was 0.914 (95% CI: 0.887–940), with sensitivity of 0.880, and specificity of 0.760 (
The AUC of ROC curve of the FTC prediction model constructed by combining predictors was 0.891 (95% CI: 0.858–0.923), with sensitivity of 0.723, and specificity of 0.914. The AUC of the prediction model constructed using scores was 0.877 (95% CI: 0.844–0.910), with sensitivity of 0.647, and specificity of 0.951 (
The AUC of ROC curve of the ATC prediction model constructed by combining predictors 0.982 (95% CI: 0.969–0.996), with sensitivity of 0.907, and specificity of 0.99. The AUC of the prediction model constructed using scores was 0.952 (95% CI: 0.917–0.987), with sensitivity of 0.930, and specificity of 0.857 (
Discussion
Thyroid nodules have a prevalence of up to 65% in the general population. Hence, appropriate ultrasonic examination is a key step to effectively monitor and manage this disease. Ultrasound examination is the first choice in the evaluation of thyroid nodules and is the primary tool for cancer risk stratification and in ascertaining the need for fine-needle biopsy [1,26]. The ACR-TIRADS risk stratification system enables assessment of all thyroid nodules [27,28]. The system is relatively simple to use and can be applied in practices with a varying number of patients with thyroid nodules as well as in practices with differing levels of expertise [29]. A comparative analysis of the 4 risk stratification systems, namely, ATA, ACR-TIRADS, EU-TIRADS, and KWAK-TIRADS, showed that ACR-TIRADS exhibited optimum diagnostic performance (AUC=0.879) and specificity [30]. The best algorithm was selected among the 5 thyroid nodule ultrasound scores (KTIRADS, ATA, AACE/ACE-AME, EU-TIRADS, and ACR-TIRADS), and the ACR-TIRADS total score demonstrated the highest accuracy, at 0.647 (95% CI: 0.625–0.669) [31]. Although the ACR-TIRADS ultrasound risk stratification system is primarily designed to detect PTC, it also appears to provide reliable recommendations for fine-needle biopsy testing in FTC [23] and MTC [25]. Jin et al [32] used a score of 4.5 as the optimal cut-off value and obtained an accuracy of 75.6%, sensitivity of 85.0%, and specificity of 71.6% in predicting malignant tumors. In our study, the specificity was comparatively higher. It has also been reported that with a cut-off value of 4, the accuracy, sensitivity, and specificity were 87.56%, 92%, and 83.49%, respectively [33]. Using ACR-TIRADS criteria, Hoang et al unanimously recommended biopsy in 55 of 100 nodules, with sensitivity, specificity, and accuracy of 87%, 51%, and 56%, respectively [34]. Considering the high relative prevalence of FTC, MTC, and undifferentiated carcinoma in our study, compared with that of other studies, the results could have differed due to interobserver heterogeneity and differences in study populations and sample sizes.
Reports have stated that fine-needle aspiration will not be performed based on the ultrasound patterns in at least 31% of FTC cases. As cytology has certain limitations, great caution should be exercised in using ultrasound as a follow-up tool for indeterminate nodules [30,35]. While fine-needle aspiration can accurately diagnose PTC, FTC is always classified in the uncertain fine-needle aspiration category [36]. Despite the presence of some malignant signs in FTC, this classification system cannot be fully applied to predict FTC [21,37]. In our study, the AUC for the diagnosis of FTC was the lowest, at 0.877 (95% CI: 0.843, 0.912), and the specificity was also only 64.8% when the cut-off score was 3.5. This result can be related to its ultrasonic features, which were found in 9.9% and 4.9% of the patients with cystic solid and hyper-/iso-echogenicity in our study, from the 4 pathological types. This observation is also in line with the claim that cystic changes are associated with FTC [21]. Although the diagnostic specificity and Youden index of ultrasound images are not high, a study has combined preoperative thyroglobulin levels and other risk factors to create a model for predicting FTC, in which the accuracy, sensitivity, and specificity were improved to 89.2%, 90.2%, and 87.7%, respectively [24]. This finding demonstrates the need for better predictive models to diagnose FTC. In addition, in our study, we found that the main features of MTC were extremely hypoechoic in 16.7% (16) and fine dot-like calcifications in 39.6% (38) of cases, which is consistent with the fact that one-third of all MTC cases analyzed by Kim et al [38] exhibited ultrasonic features similar to the benign nodules. Furthermore, it has been reported that the sensitivity of ultrasound for the diagnosis of MTC was 75.3%, specificity was 93.1%, and the overall accuracy was 80.4% [39]. Hence, the sensitivity was comparable to that of our study, but the accuracy in our study was higher, suggesting that our system was reliable in detecting MTC. In our study, we used multiple indicators to build a diagnostic MTC prediction model and used the ACR-TIRADS total score to build a prediction model. The AUC was 0.939 and 0.914, the sensitivity was 0.975 and 0.880, and the specificity was 0.729 and 0.760, respectively. The prediction model can produce a higher sensitivity in diagnosing MTC and was more conducive to screening out positive nodules; however, the specificity was low, which can be due to different sample sizes and selection bias.
ATC is an aggressive but rare malignant thyroid tumor that accounts for only 1% to 2% of all thyroid cancers [40]. However, this subtype is associated with a poor prognosis, and the therapeutic options are limited [41]. Survival remains low regardless of surgery, radiotherapy with or without chemotherapy, or palliative and symptomatic treatment [42,43], and the underlying mechanism is variable, from signaling pathways to target gene regulatory networks [44–46]. Nearly 50% of ATCs occur pathophysiologically in the context of preexisting or concurrent differentiated thyroid carcinoma, suggesting that a significant proportion of ATC is caused by the dedifferentiation of preexisting differentiated thyroid carcinoma into a more aggressive phenotype. Hence, the diagnostic efficacy of ATC on a graded scale is close to that of differentiated thyroid carcinoma, which is in line with the results of the present study. The main features of ATC in our study were extracapsular thyroid invasion in 47.6% (10) of cases, which is consistent with the fact that patients with ATC usually present with a rapidly enlarging neck mass and clinical symptoms related to the compression of local structures (eg, neck vessels, esophagus, or trachea) [47]. Also, some studies have shown that in the evaluation of the accuracy of ACR-TIRADS, the diagnostic accuracy of PTC is the highest, although the reliability of detecting MTC, FTC, and other malignant tumors is not high. It has therefore been suggested to modify the mode and cut-off point of fine-needle aspiration or to combine ultrasound with other techniques [48]. Multimodal ultrasound examinations, such as combined contrast and elastography, can be recommended clinically for patients with suspected malignant thyroid nodules [49], or other techniques, such as molecular gene modification can be recommended [50]. In summary, our study suggested a valuable diagnostic efficiency of PTC based on the ACR-TIRADS score, which will be very useful in the clinic for the pathological classification of malignant nodules in patients.
Conclusions
Thyroid ultrasound examination based on the ACR-TIRADS score is helpful in the diagnosis of benign and malignant thyroid nodules, and has certain diagnostic value for different pathological types of malignant thyroid nodules. Although this score is more conducive to the diagnosis of PTC and ATC, the prediction model constructed through multiple indicators has better diagnostic performance. The prediction model for diagnosing FTC and MTC constructed through multiple indicators was compared with the model constructed based on the ACR-TIRADS total score. The diagnostic efficiency was improved, but the difference was not statistically significant. Due to the imbalance of data, prospective, multi-center studies are needed to verify the results obtained.
Figures
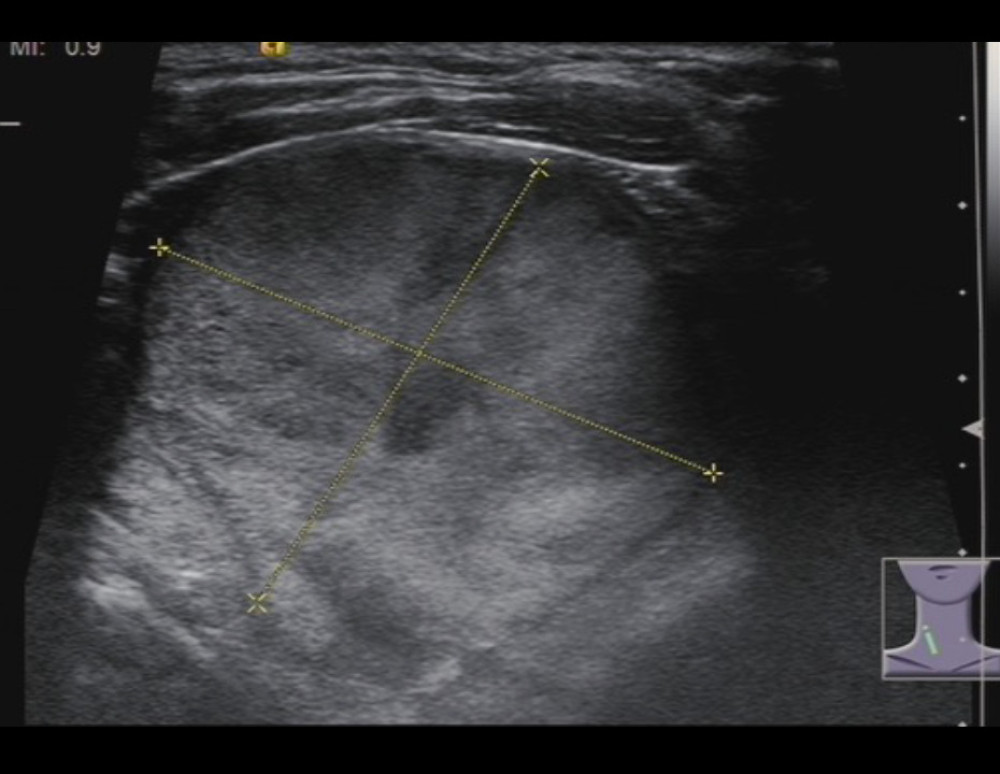 Figure 1. A 77-year-old woman with follicular thyroid carcinoma: solid (2 points), isoechoic (1 point), wider-than-tall (0 points), extra-thyroidal extension (3 points), none echogenic foci (0 points), total score 6, TR4 class.
Figure 1. A 77-year-old woman with follicular thyroid carcinoma: solid (2 points), isoechoic (1 point), wider-than-tall (0 points), extra-thyroidal extension (3 points), none echogenic foci (0 points), total score 6, TR4 class. 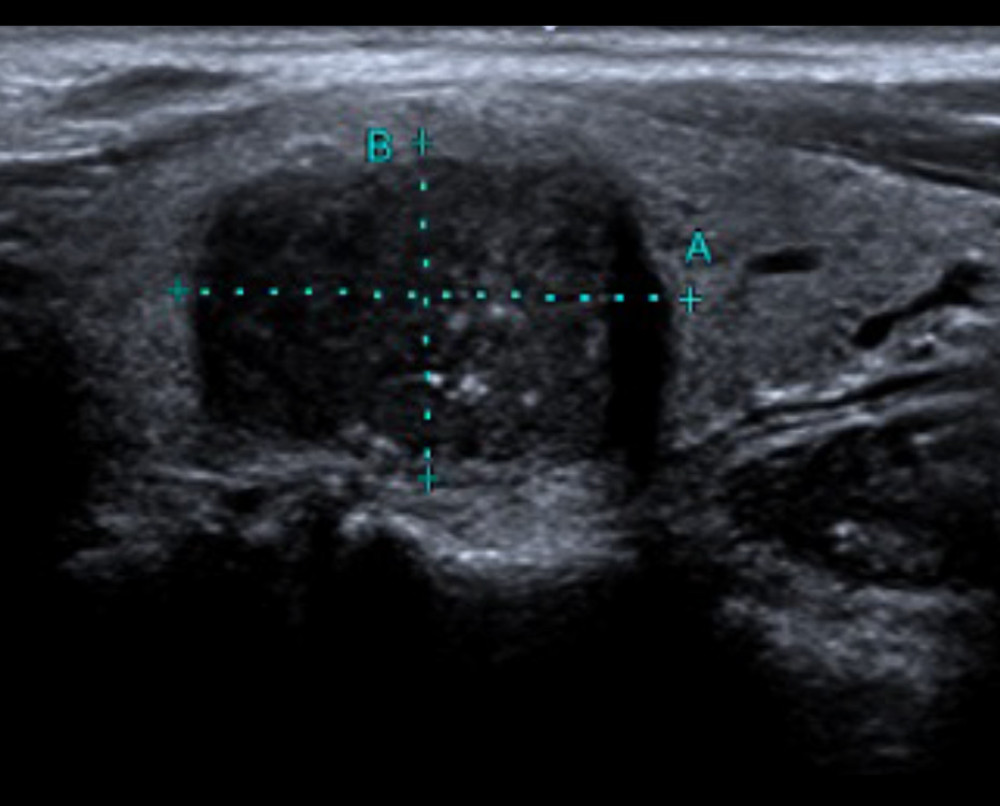 Figure 2. A 43-year-old man with medullary thyroid carcinoma: solid (2 points), very hypoechoic (3 points), wider-than-tall (0 points), borderline irregular (2 points), punctate echogenic foci (3 points), total score 10, TR5 class.
Figure 2. A 43-year-old man with medullary thyroid carcinoma: solid (2 points), very hypoechoic (3 points), wider-than-tall (0 points), borderline irregular (2 points), punctate echogenic foci (3 points), total score 10, TR5 class. 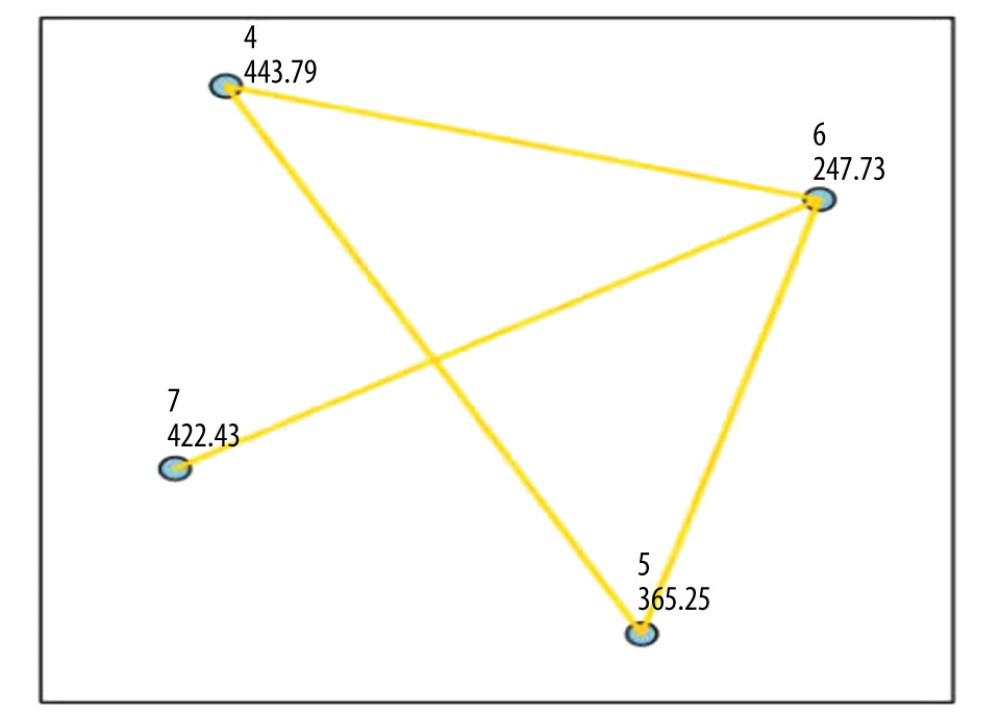 Figure 3. Comparison of the 4 pathological types of thyroid malignant nodules. 4=papillary thyroid carcinoma, 5=medullary thyroid carcinoma, 6=follicular thyroid carcinoma, 7=anaplastic thyroid carcinoma.
Figure 3. Comparison of the 4 pathological types of thyroid malignant nodules. 4=papillary thyroid carcinoma, 5=medullary thyroid carcinoma, 6=follicular thyroid carcinoma, 7=anaplastic thyroid carcinoma. 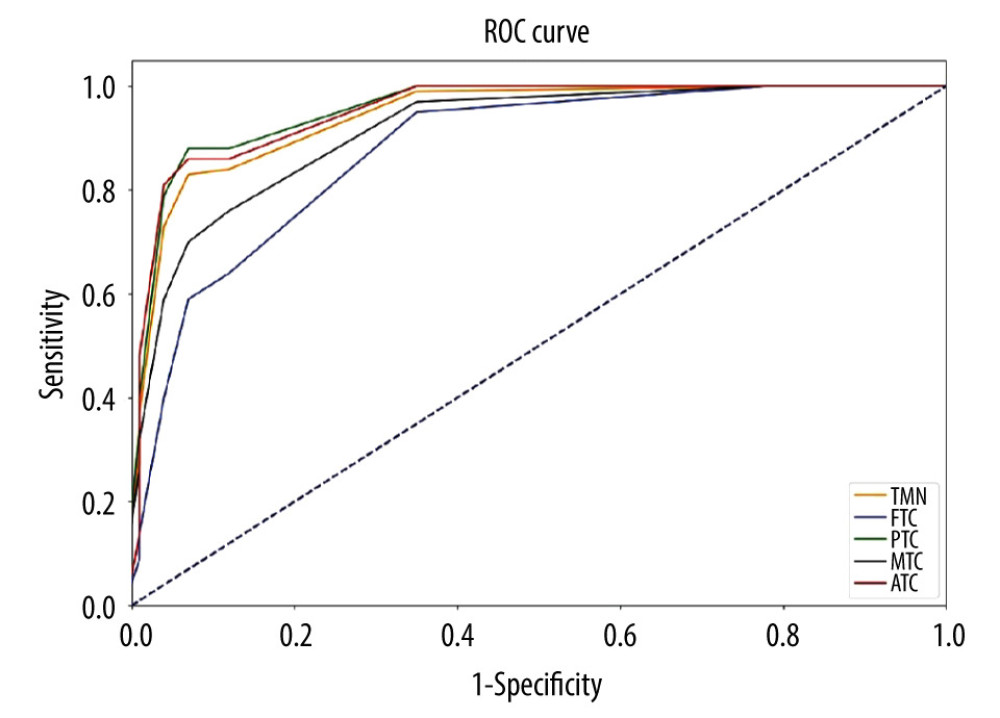 Figure 4. Comparison of the ROC curves of thyroid malignant nodules and different pathological types.TMN – total malignant nodules; FTC – follicular thyroid carcinoma; PTC – papillary thyroid carcinoma; MTC – medullary thyroid carcinoma; ATC – anaplastic thyroid carcinoma.
Figure 4. Comparison of the ROC curves of thyroid malignant nodules and different pathological types.TMN – total malignant nodules; FTC – follicular thyroid carcinoma; PTC – papillary thyroid carcinoma; MTC – medullary thyroid carcinoma; ATC – anaplastic thyroid carcinoma. Tables
Table 1. Comparison of general data and ultrasonic characteristics of benign and malignant thyroid nodules.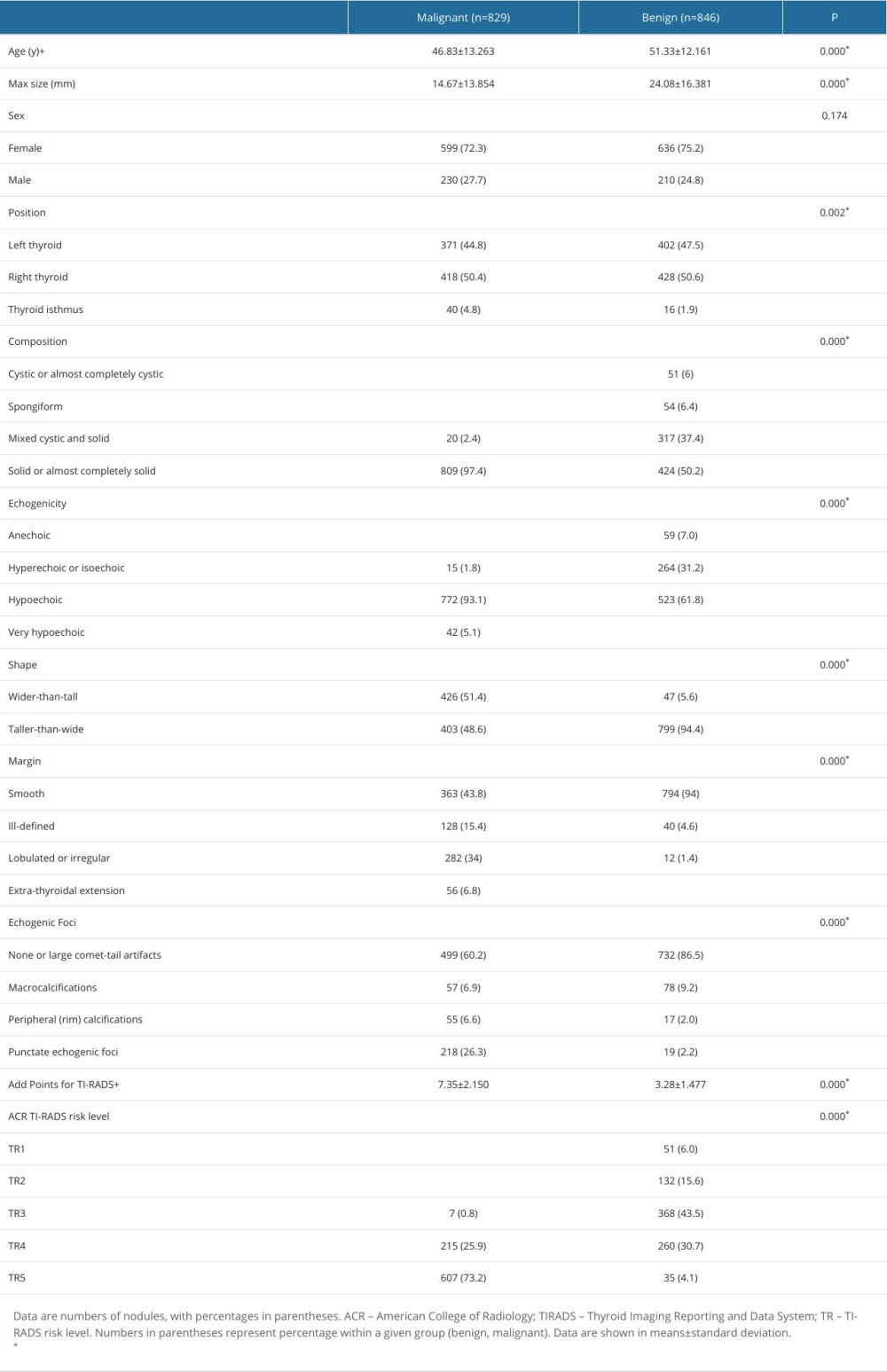 Table 2. Distribution of the ultrasonographic characteristics of 4 pathological types of thyroid malignant nodules.
Table 2. Distribution of the ultrasonographic characteristics of 4 pathological types of thyroid malignant nodules.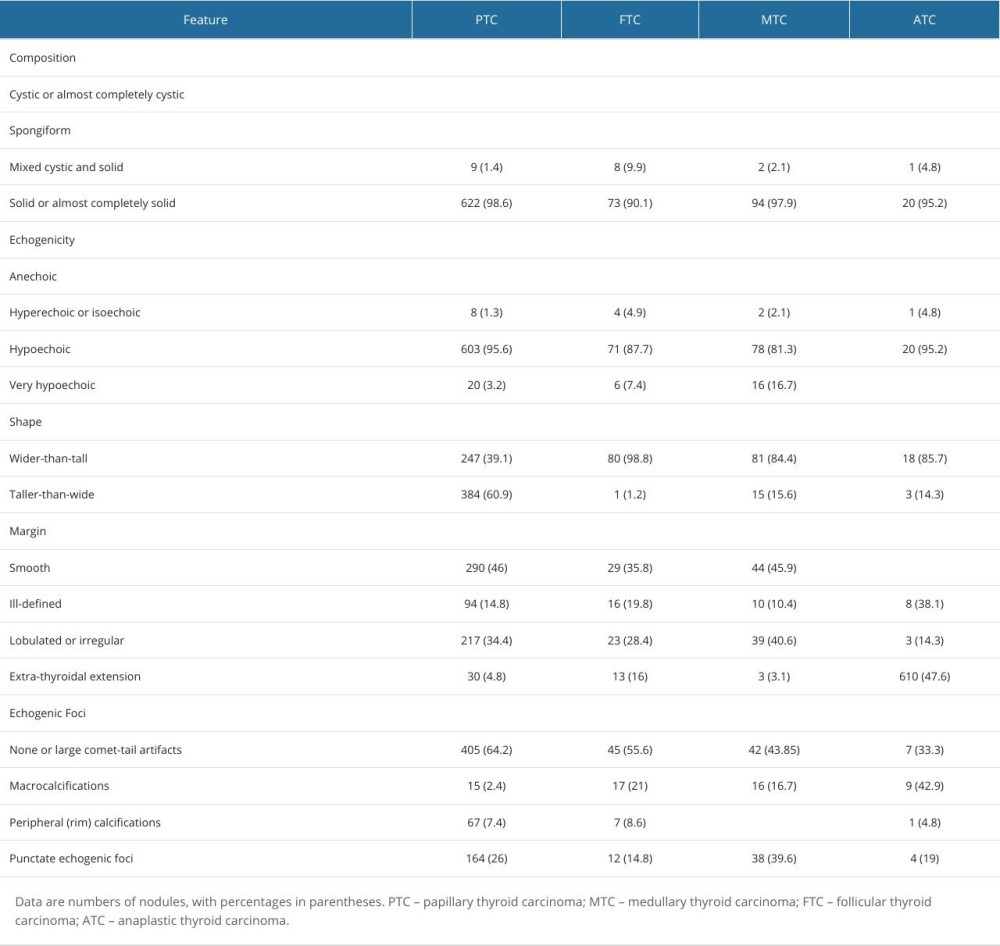 Table 3. ACR score distribution of 4 pathological types of thyroid malignant nodules.
Table 3. ACR score distribution of 4 pathological types of thyroid malignant nodules.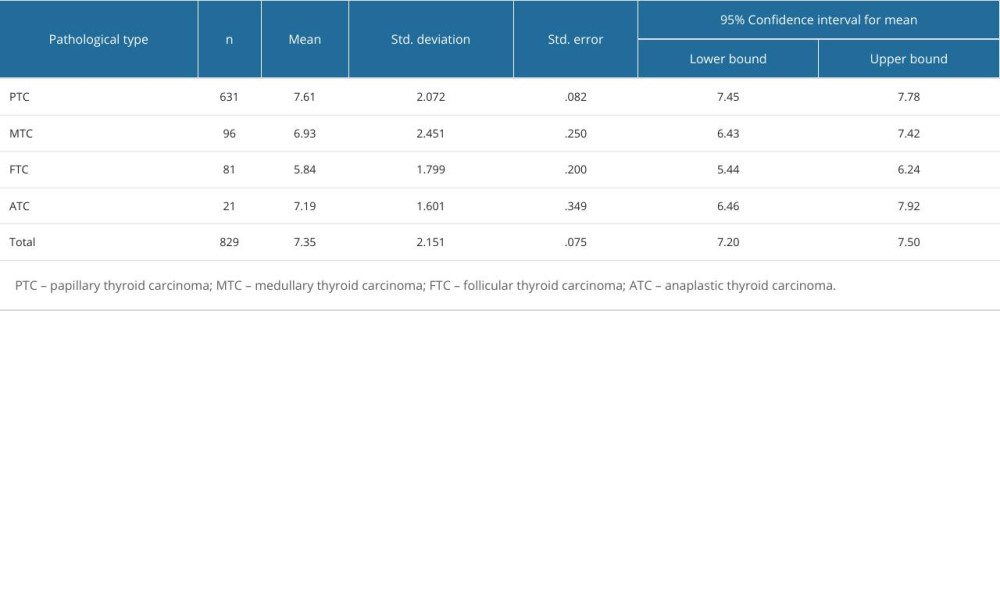 Table 4. Pairwise comparison of thyroid malignant nodules based on ACR-TIRADS score between groups.
Table 4. Pairwise comparison of thyroid malignant nodules based on ACR-TIRADS score between groups.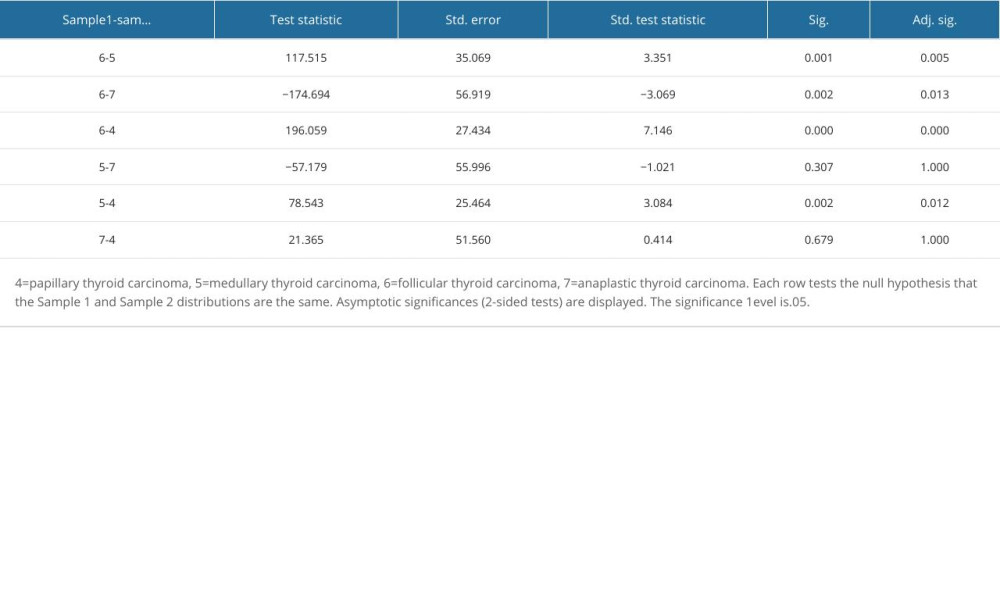 Table 5. Diagnostic efficacy of ACR-TIRADS score for different pathologic types of thyroid malignant nodules.
Table 5. Diagnostic efficacy of ACR-TIRADS score for different pathologic types of thyroid malignant nodules.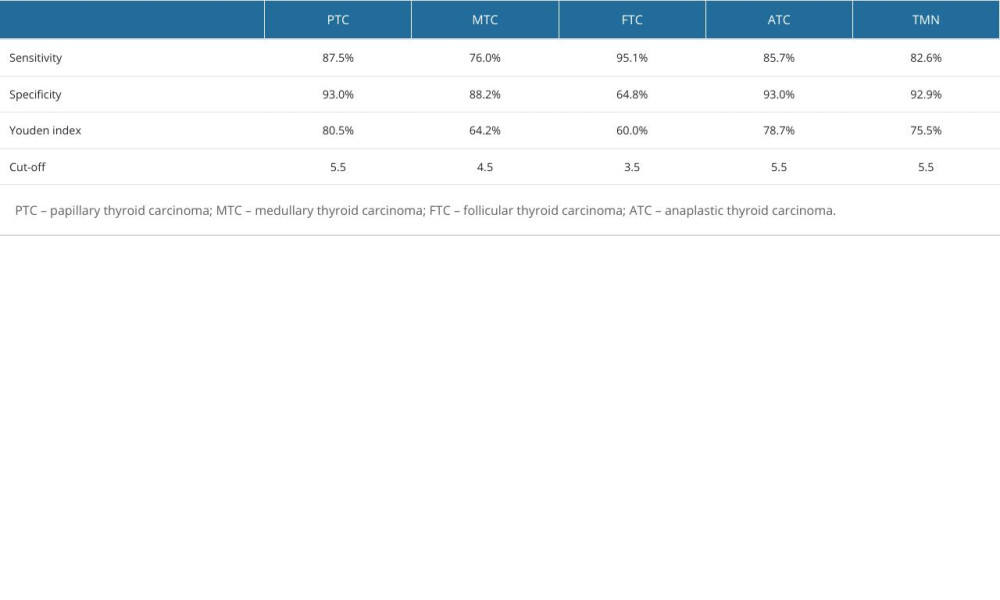 Table 6. Logistic regression analysis of predictors of thyroid malignant nodules.
Table 6. Logistic regression analysis of predictors of thyroid malignant nodules.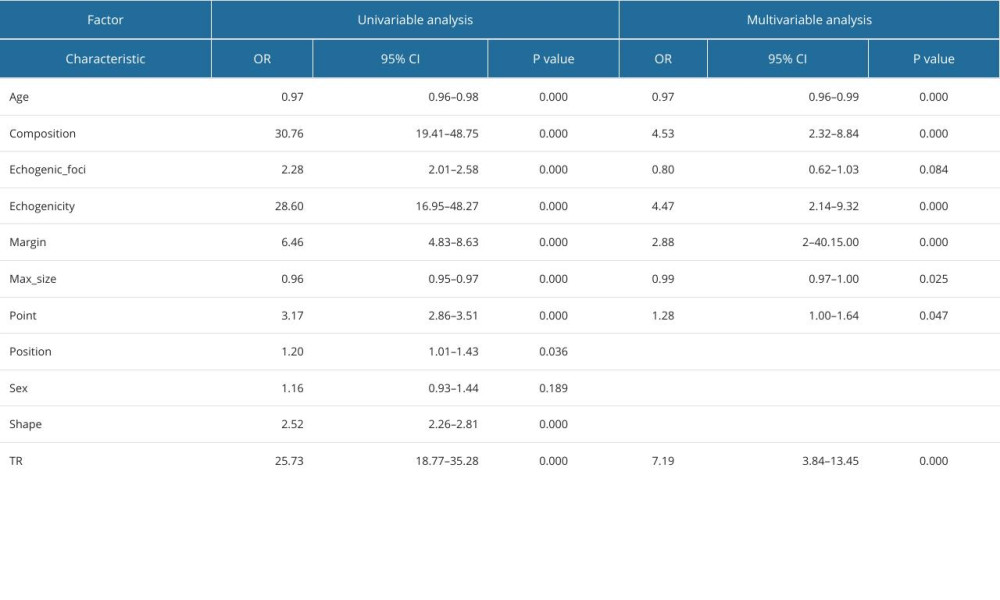 Table 7. Logistic regression analysis of predictors of papillary thyroid carcinoma.
Table 7. Logistic regression analysis of predictors of papillary thyroid carcinoma.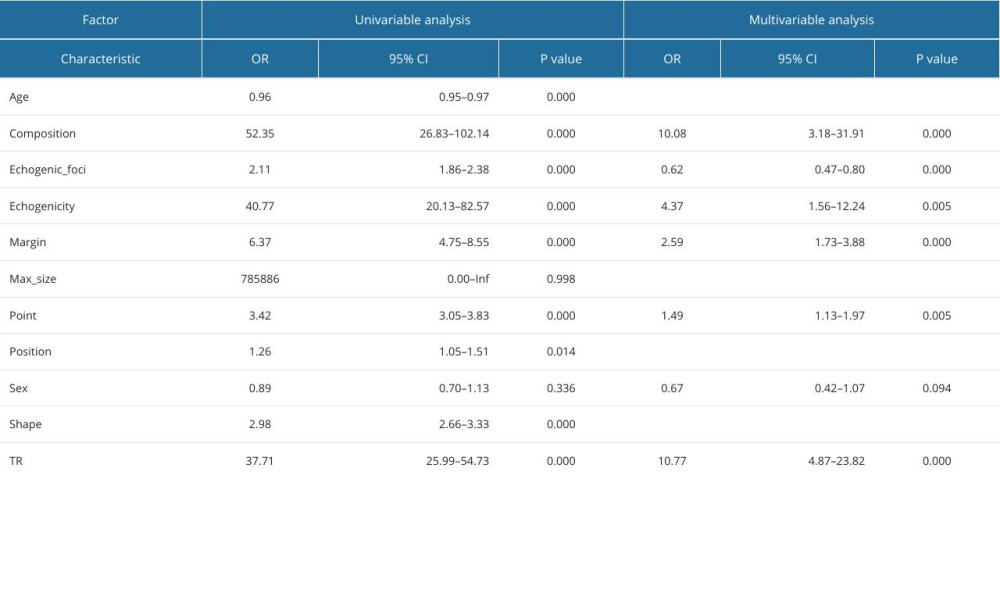 Table 8. Logistic regression analysis of predictors of medullary thyroid carcinoma.
Table 8. Logistic regression analysis of predictors of medullary thyroid carcinoma.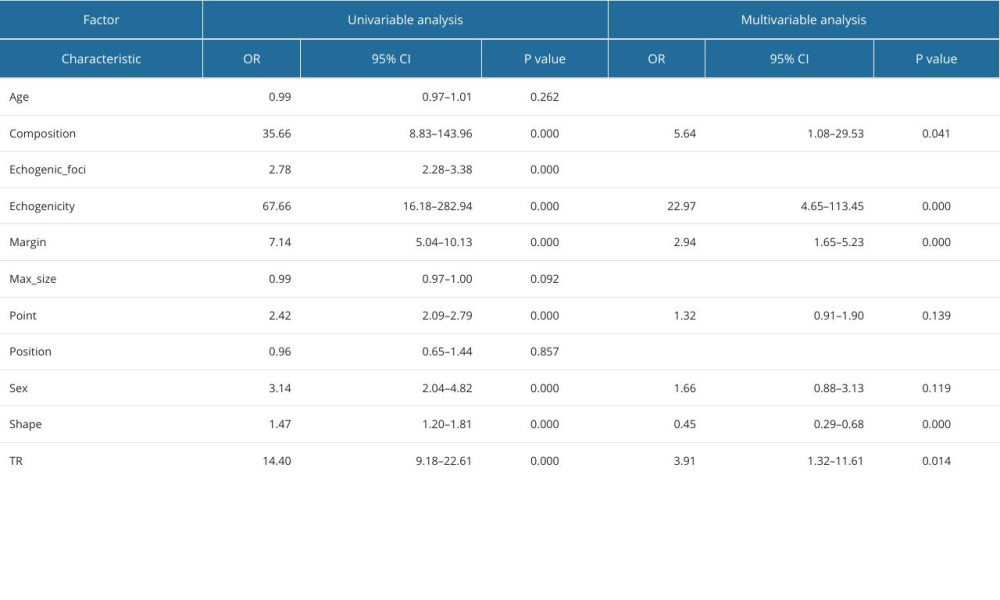 Table 9. Logistic regression analysis of predictors of follicular thyroid carcinoma.
Table 9. Logistic regression analysis of predictors of follicular thyroid carcinoma.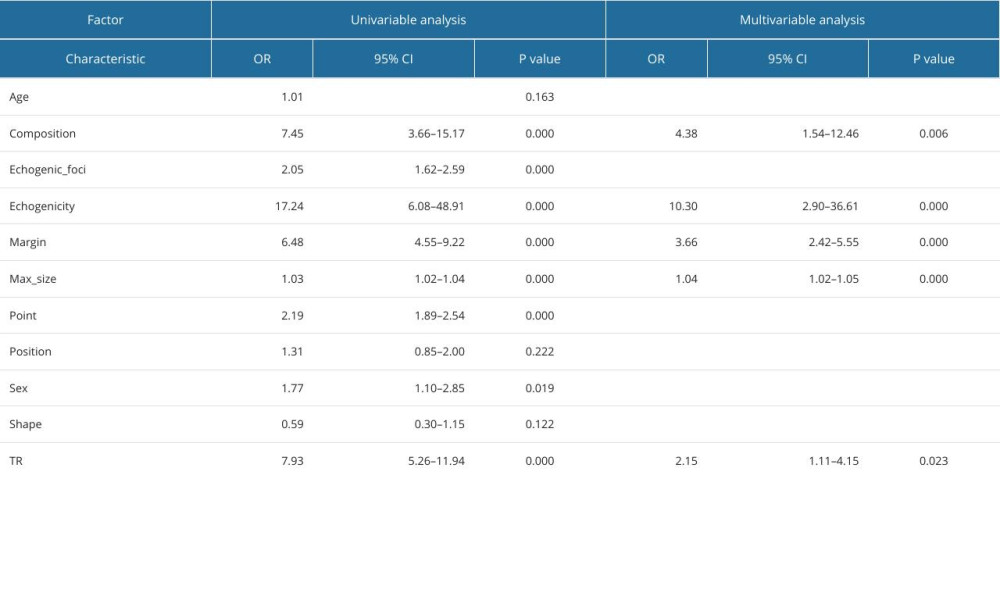 Table 10. Logistic regression analysis of predictors of anaplastic thyroid carcinoma.
Table 10. Logistic regression analysis of predictors of anaplastic thyroid carcinoma.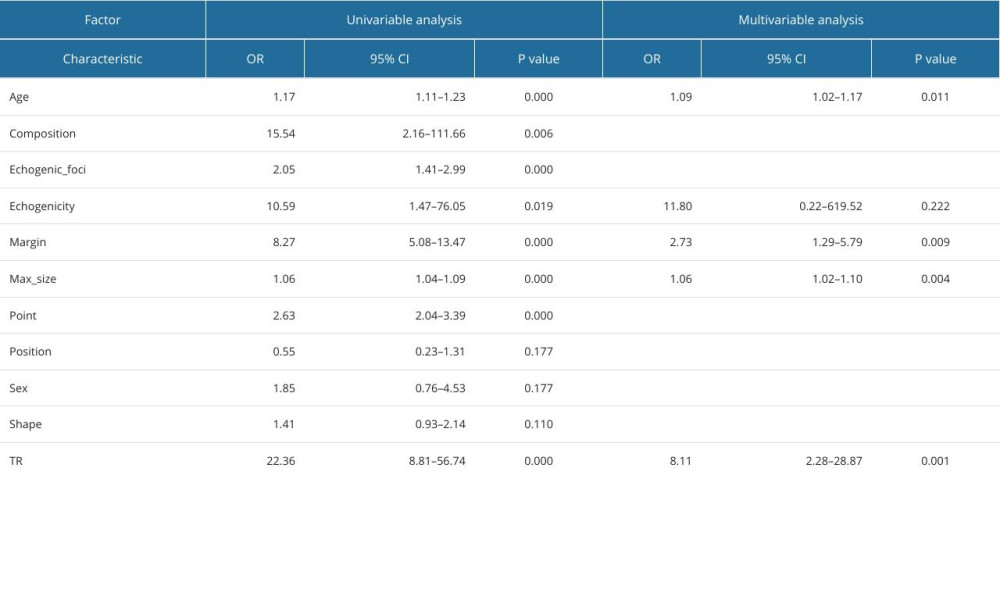
References
1. Durante C, Grani G, Lamartina L, The diagnosis and management of thyroid nodules: A review: JAMA, 2018; 319(9); 914-24
2. Cai M, Chen L, Shui L, Lv X, Wang H, Explore the diagnostic performance of 2020 Chinese Thyroid Imaging Reporting and Data Systems by comparing with the 2017 ACR-TIRADS guidelines: A single-center study: Endocrine, 2023; 80(2); 399-407
3. Lim H, Devesa SS, Sosa JA, Trends in thyroid cancer incidence and mortality in the United States, 1974–2013: JAMA, 2017; 317(13); 1338-48
4. Grani G, Sponziello M, Pecce V, Contemporary thyroid nodule evaluation and management: J Clin Endocrinol Metab, 2020; 105(9); 2869-83
5. Smith-Bindman R, Lebda P, Feldstein VA, Risk of thyroid cancer based on thyroid ultrasound imaging characteristics: Results of a population-based study: JAMA Intern Med, 2013; 173(19); 1788-96
6. Jiang L, Zhang D, Chen YN, The value of conventional ultrasound combined with superb microvascular imaging and color Doppler flow imaging in the diagnosis of thyroid malignant nodules: A systematic review and meta-analysis: Front Endocrinol (Lausanne), 2023; 14; 1182259
7. Huang J, Zhao J, Quantitative diagnosis progress of ultrasound imaging technology in thyroid diffuse diseases: Diagnostics (Basel), 2023; 13(4); 700
8. Floridi C, Cellina M, Buccimazza G, Ultrasound imaging classifications of thyroid nodules for malignancy risk stratification and clinical management: state of the art: Gland Surg, 2019; 8(Suppl 3); S233-S44
9. Lauria Pantano A, Maddaloni E, Briganti SI, Differences between ATA, AACE/ACE/AME and ACR TI-RADS ultrasound classifications performance in identifying cytological high-risk thyroid nodules: Eur J Endocrinol, 2018; 178(6); 595-603
10. Kabootari M, Habibi Tirtashi R, Zadeh-Vakili A, RET/PTC rearrangement in papillary thyroid carcinoma arising in malignant struma ovarii with abdominal wall metastasis and cervical thyroid gland: A case report and review of the literature: Thyroid Res, 2023; 16(1); 39
11. Erdogan AM, Alagoz S, Bal KK, The relationship between American College of Radiology Thyroid Imaging Reporting and Data System (ACR TIRADS) and Fine Needle Aspiration Cytology (FNAC); in geriatric thyroid pathologies: Indian J Otolaryngol Head Neck Surg, 2023; 75(2); 318-21
12. Chen F, Sun Y, Chen G, The Diagnostic Efficacy of the American College of Radiology (ACR) Thyroid Imaging Report and Data System (TI-RADS) and the American Thyroid Association (ATA) Risk Stratification Systems for Thyroid Nodules: Comput Math Methods Med, 2022; 2022; 9995962
13. Tessler FN, Middleton WD, Grant EG, ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee: J Am Coll Radiol, 2017; 14(5); 587-95
14. Koseoglu Atilla FD, Ozgen Saydam B, Erarslan NA, Does the ACR TI-RADS scoring allow us to safely avoid unnecessary thyroid biopsy? Single center analysis in a large cohort: Endocrine, 2018; 61(3); 398-402
15. Grani G, Sponziello M, Pecce V, Contemporary thyroid nodule evaluation and management: J Clin Endocrinol Metab, 2020; 105(9); 2869-83
16. Griffin AS, Mitsky J, Rawal U, Improved quality of thyroid ultrasound reports after implementation of the ACR Thyroid Imaging Reporting and Data System Nodule Lexicon and Risk Stratification System: J Am Coll Radiol, 2018; 15(5); 743-48
17. Rocha TG, Rosario PW, Silva AL, Thyroid imaging reporting and data system (TI-RADS) of the American College of Radiology (ACR) for predicting malignancy in thyroid nodules >1 cm with indeterminate cytology: Diagn Cytopathol, 2019; 47(5); 523-25
18. Abou Shaar B, Meteb M, Awad El-Karim G, Almalki Y, Reducing the number of unnecessary thyroid nodule biopsies with the American College of Radiology (ACR) Thyroid Imaging Reporting and Data System (TI-RADS): Cureus, 2022; 14(3); e23118
19. Middleton WD, Teefey SA, Reading CC, Comparison of Performance Characteristics of American College of Radiology TI-RADS, Korean Society of Thyroid Radiology TIRADS, and American Thyroid Association Guidelines: Am J Roentgenol, 2018; 210(5); 1148-54
20. Yim Y, Na DG, Ha EJ, Concordance of Three International Guidelines for Thyroid Nodules Classified by ultrasonography and diagnostic performance of biopsy criteria: Korean J Radiol, 2020; 21(1); 108-16
21. Ou D, Yao J, Jin J, Ultrasonic identification and regression analysis of 294 thyroid follicular tumors: J Cancer Res Ther, 2020; 16(5); 1056-62
22. Wright K, Brandler TC, Fisher JC, The clinical significance of the American College of Radiology (ACR) Thyroid Imaging Reporting and Data System (TI-RADS) category 5 thyroid nodules: Not as risky as we think?: Surgery, 2023; 173(1); 239-45
23. Castellana M, Piccardo A, Virili C, Can ultrasound systems for risk stratification of thyroid nodules identify follicular carcinoma?: Cancer Cytopathol, 2020; 128(4); 250-59
24. Yu Q, Liu K, Xie C, Development and validation of a preoperative prediction model for follicular thyroid carcinoma: Clin Endocrinol (Oxf), 2019; 91(2); 348-55
25. Valderrabano P, Klippenstein DL, Tourtelot JB, New American Thyroid Association sonographic patterns for thyroid nodules perform well in medullary thyroid carcinoma: Institutional experience, systematic review, and meta-analysis: Thyroid, 2016; 26(8); 1093-100
26. Melany M, Chen S, Thyroid cancer: Ultrasound imaging and fine-needle aspiration biopsy: Endocrinol Metab Clin North Am, 2017; 46(3); 691-711
27. Fu P, Chen W, Cui LGApplicational value of 2017 ACR TI-RADS stratification in diagnosing thyroid nodules: Beijing Da Xue Xue Bao Yi Xue Ban, 2019; 51(6); 1067-70 in Chinese
28. Juma SN, Liao J, Huang Y, Osteoarthritis versus psoriasis arthritis: Physiopathology, cellular signaling, and therapeutic strategies: Genes Dis, 2023; 11(3); 100986
29. Middleton WD, Teefey SA, Reading CC, Multiinstitutional analysis of thyroid nodule risk stratification using the American College of Radiology Thyroid Imaging Reporting and Data System: Am J Roentgenol, 2017; 208(6); 1331-41
30. Shen Y, Liu M, He J, Comparison of different risk-stratification systems for the diagnosis of benign and malignant thyroid nodules: Front Oncol, 2019; 9; 378
31. Sparano C, Verdiani V, Pupilli C, Choosing the best algorithm among five thyroid nodule ultrasound scores: From performance to cytology sparing-a single-center retrospective study in a large cohort: Eur Radiol, 2021; 31(8); 5689-98
32. Jin ZQ, Yu HZ, Mo CJ, Su RQ, Clinical study of the prediction of malignancy in thyroid nodules: Modified Score versus 2017 American College of Radiology’s Thyroid Imaging Reporting and Data System Ultrasound Lexicon: Ultrasound Med Biol, 2019; 45(7); 1627-37
33. Ye H, Hang J, Chen X, An intelligent platform for ultrasound diagnosis of thyroid nodules: Sci Rep, 2020; 10(1); 13223
34. Hoang JK, Middleton WD, Farjat AE, Reduction in thyroid nodule biopsies and improved accuracy with American College of Radiology Thyroid Imaging Reporting and Data System: Radiology, 2018; 287(1); 185-93
35. Huang Y, Liao J, Vlashi R, Chen G, Focal adhesion kinase (FAK): Its structure, characteristics, and signaling in skeletal system: Cell Signal, 2023; 111; 110852
36. Grani G, Lamartina L, Durante C, Follicular thyroid cancer and Hürthle cell carcinoma: Challenges in diagnosis, treatment, and clinical management: Lancet Diabetes Endocrinol, 2018; 6(6); 500-14
37. Vlashi R, Zhang X, Wu M, Chen G, Wnt signaling: Essential roles in osteoblast differentiation, bone metabolism and therapeutic implications for bone and skeletal disorders: Genes Dis, 2023; 10(4); 1291-317
38. Kim C, Baek JH, Ha E, Ultrasonography features of medullary thyroid cancer as predictors of its biological behavior: Acta Radiol, 2017; 58(4); 414-22
39. Wang L, Kou H, Chen W, The diagnostic value of ultrasound in medullary thyroid carcinoma: A comparison with computed tomography: Technol Cancer Res Treat, 2020; 19; 1533033820905832
40. Mao Y, Xing M, Recent incidences and differential trends of thyroid cancer in the USA: Endocr Relat Cancer, 2016; 23(4); 313-22
41. Haddad RI, Nasr C, Bischoff L, NCCN Guidelines Insights: Thyroid carcinoma, Version 2.2018: J Natl Compr Canc Netw, 2018; 16(12); 1429-40
42. N Nachalon Y, Stern-Shavit S, Bachar G, Aggressive palliation and survival in anaplastic thyroid carcinoma: JAMA Otolaryngol Head Neck Surg, 2015; 141(12); 1128-32
43. Hu S, Chen S, Zeng H, Ap-2beta regulates cranial osteogenic potential via the activation of Wnt/beta-catenin signaling pathway: Dev Biol, 2023; 501; 81-91
44. Liao J, Huang Y, Wang Q, Gene regulatory network from cranial neural crest cells to osteoblast differentiation and calvarial bone development: Cell Mol Life Sci, 2022; 79(3); 158
45. Chen G, Xu H, Yao Y, BMP Signaling in the development and regeneration of cranium bones and maintenance of calvarial stem cells: Front Cell Dev Biol, 2020; 8; 135
46. Chen G, Yao Y, Xu G, Zhang X, Regional difference in microRNA regulation in the skull vault: Dev Dyn, 2019; 248(10); 1009-19
47. Nel CJ, van Heerden JA, Goellner JR, Anaplastic carcinoma of the thyroid: A clinicopathologic study of 82 cases: Mayo Clin Proc, 1985; 60(1); 51-58
48. Trimboli P, Castellana M, Piccardo A, The ultrasound risk stratification systems for thyroid nodule have been evaluated against papillary carcinoma. A meta-analysis: Rev Endocr Metab Disord, 2021; 22(2); 453-60
49. Wang J, He X, Ma L, Multimode ultrasonic technique is recommended for the differential diagnosis of thyroid cancer: Peer J, 2020; 8; e9112
50. Vlashi R, Zhang X, Li H, Chen G, Potential therapeutic strategies for osteoarthritis via CRISPR/Cas9 mediated gene editing: Rev Endocr Metab Disord, 2023 [Online ahead of print] [Erratum in: Rev Endocr Metab Disord. 2023 [Online ahead of print]
Figures
 Figure 1. A 77-year-old woman with follicular thyroid carcinoma: solid (2 points), isoechoic (1 point), wider-than-tall (0 points), extra-thyroidal extension (3 points), none echogenic foci (0 points), total score 6, TR4 class.
Figure 1. A 77-year-old woman with follicular thyroid carcinoma: solid (2 points), isoechoic (1 point), wider-than-tall (0 points), extra-thyroidal extension (3 points), none echogenic foci (0 points), total score 6, TR4 class. Figure 2. A 43-year-old man with medullary thyroid carcinoma: solid (2 points), very hypoechoic (3 points), wider-than-tall (0 points), borderline irregular (2 points), punctate echogenic foci (3 points), total score 10, TR5 class.
Figure 2. A 43-year-old man with medullary thyroid carcinoma: solid (2 points), very hypoechoic (3 points), wider-than-tall (0 points), borderline irregular (2 points), punctate echogenic foci (3 points), total score 10, TR5 class. Figure 3. Comparison of the 4 pathological types of thyroid malignant nodules. 4=papillary thyroid carcinoma, 5=medullary thyroid carcinoma, 6=follicular thyroid carcinoma, 7=anaplastic thyroid carcinoma.
Figure 3. Comparison of the 4 pathological types of thyroid malignant nodules. 4=papillary thyroid carcinoma, 5=medullary thyroid carcinoma, 6=follicular thyroid carcinoma, 7=anaplastic thyroid carcinoma. Figure 4. Comparison of the ROC curves of thyroid malignant nodules and different pathological types.TMN – total malignant nodules; FTC – follicular thyroid carcinoma; PTC – papillary thyroid carcinoma; MTC – medullary thyroid carcinoma; ATC – anaplastic thyroid carcinoma.
Figure 4. Comparison of the ROC curves of thyroid malignant nodules and different pathological types.TMN – total malignant nodules; FTC – follicular thyroid carcinoma; PTC – papillary thyroid carcinoma; MTC – medullary thyroid carcinoma; ATC – anaplastic thyroid carcinoma. Tables
 Table 1. Comparison of general data and ultrasonic characteristics of benign and malignant thyroid nodules.
Table 1. Comparison of general data and ultrasonic characteristics of benign and malignant thyroid nodules. Table 2. Distribution of the ultrasonographic characteristics of 4 pathological types of thyroid malignant nodules.
Table 2. Distribution of the ultrasonographic characteristics of 4 pathological types of thyroid malignant nodules. Table 3. ACR score distribution of 4 pathological types of thyroid malignant nodules.
Table 3. ACR score distribution of 4 pathological types of thyroid malignant nodules. Table 4. Pairwise comparison of thyroid malignant nodules based on ACR-TIRADS score between groups.
Table 4. Pairwise comparison of thyroid malignant nodules based on ACR-TIRADS score between groups. Table 5. Diagnostic efficacy of ACR-TIRADS score for different pathologic types of thyroid malignant nodules.
Table 5. Diagnostic efficacy of ACR-TIRADS score for different pathologic types of thyroid malignant nodules. Table 6. Logistic regression analysis of predictors of thyroid malignant nodules.
Table 6. Logistic regression analysis of predictors of thyroid malignant nodules. Table 7. Logistic regression analysis of predictors of papillary thyroid carcinoma.
Table 7. Logistic regression analysis of predictors of papillary thyroid carcinoma. Table 8. Logistic regression analysis of predictors of medullary thyroid carcinoma.
Table 8. Logistic regression analysis of predictors of medullary thyroid carcinoma. Table 9. Logistic regression analysis of predictors of follicular thyroid carcinoma.
Table 9. Logistic regression analysis of predictors of follicular thyroid carcinoma. Table 10. Logistic regression analysis of predictors of anaplastic thyroid carcinoma.
Table 10. Logistic regression analysis of predictors of anaplastic thyroid carcinoma. Table 1. Comparison of general data and ultrasonic characteristics of benign and malignant thyroid nodules.
Table 1. Comparison of general data and ultrasonic characteristics of benign and malignant thyroid nodules. Table 2. Distribution of the ultrasonographic characteristics of 4 pathological types of thyroid malignant nodules.
Table 2. Distribution of the ultrasonographic characteristics of 4 pathological types of thyroid malignant nodules. Table 3. ACR score distribution of 4 pathological types of thyroid malignant nodules.
Table 3. ACR score distribution of 4 pathological types of thyroid malignant nodules. Table 4. Pairwise comparison of thyroid malignant nodules based on ACR-TIRADS score between groups.
Table 4. Pairwise comparison of thyroid malignant nodules based on ACR-TIRADS score between groups. Table 5. Diagnostic efficacy of ACR-TIRADS score for different pathologic types of thyroid malignant nodules.
Table 5. Diagnostic efficacy of ACR-TIRADS score for different pathologic types of thyroid malignant nodules. Table 6. Logistic regression analysis of predictors of thyroid malignant nodules.
Table 6. Logistic regression analysis of predictors of thyroid malignant nodules. Table 7. Logistic regression analysis of predictors of papillary thyroid carcinoma.
Table 7. Logistic regression analysis of predictors of papillary thyroid carcinoma. Table 8. Logistic regression analysis of predictors of medullary thyroid carcinoma.
Table 8. Logistic regression analysis of predictors of medullary thyroid carcinoma. Table 9. Logistic regression analysis of predictors of follicular thyroid carcinoma.
Table 9. Logistic regression analysis of predictors of follicular thyroid carcinoma. Table 10. Logistic regression analysis of predictors of anaplastic thyroid carcinoma.
Table 10. Logistic regression analysis of predictors of anaplastic thyroid carcinoma. In Press
Clinical Research
Comparison of Fentanyl, Ketamine, and Lidocaine Combined with Propofol Anesthesia in Patients with Crohn Di...Med Sci Monit In Press; DOI: 10.12659/MSM.944116
Database Analysis
ANXA5: A Key Regulator of Immune Cell Infiltration in Hepatocellular CarcinomaMed Sci Monit In Press; DOI: 10.12659/MSM.943523
Laboratory Research
Effect of Dentin Contamination with Hemostatic Agents and Cleaning Techniques on Bonding with Self-Adhesive...Med Sci Monit In Press; DOI: 10.12659/MSM.943353
Clinical Research
Undiagnosed and Untreated Peripheral Complications of Diabetes: Findings from a Pilot Study on Diabetes-Rel...Med Sci Monit In Press; DOI: 10.12659/MSM.944239
Most Viewed Current Articles
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








