13 April 2024: Clinical Research
Effect of Nasal Continuous Positive Airway Pressure on Retinopathy of Prematurity in Preterm Newborns: A Comparative Analysis with Mechanical Ventilation and High-Flow Nasal Cannula Therapy
Daniela M. Cioboata12ABDG, Aniko M. Manea12BDE*, Oana C. Costescu12AC, Florina M. Doandes12DF, Timea E. Brandibur12BF, Nicoleta Lungu12A, Mihai Dinu3C, Florina Stoica4A, Radu E. Iacob5B, Marioara Boia12ADEDOI: 10.12659/MSM.943486
Med Sci Monit 2024; 30:e943486
Abstract
BACKGROUND: Retinopathy of prematurity (ROP), originally described as retrolental fibroplasia, represents an abnormal growth of blood vessels in the premature retina that can occur in response to oxygen therapy. The association between ROP and invasive mechanical ventilation has been widely studied in the literature; however, the relationships between different types of ventilation and ROP have not been as well documented. This study aimed to compare the association of ROP incidence with mechanical ventilation (MV), nasal continuous positive airway pressure (nCPAP), and high-flow nasal cannula (HFNC) therapies in 130 pre-term infants with gestational ages <32 weeks.
MATERIAL AND METHODS: The study includes 130 premature newborns, out of which 54 underwent MV therapy, either alone or in combination with nCPAP or HFNC therapy, 63 underwent nCPAP therapy, either alone or in combination with MV or HFNC therapy, and 23 underwent HFNC therapy, either alone or in combination with MV or nCPAP therapy. The relationships between ROP and the 3 types of ventilation were analyzed by univariate followed by multivariate logistic regression.
RESULTS: When adjusting for covariates, only nCPAP and birth weight were significantly associated with ROP, the former being a strong risk factor, with an adjusted odds ratio (AOR) of 7.264 (95% CI, 2.622-20.120; P<0.001), and the latter being a weak protective factor, with an AOR of 0.998 (95% CI, 0.996-0.999; P<0.05).
CONCLUSIONS: The results showed nCPAP was a strong ROP risk factor, birth weight was a weak ROP protective factor, and MV and HFNC were not significantly associated with increased ROP risk.
Keywords: Continuous Positive Airway Pressure, Retinopathy of Prematurity, noninvasive ventilation, Respiration, Artificial, Infant, Premature, Respiratory Distress Syndrome In Premature Infants
Introduction
The introduction of mechanical ventilation (MV) in 1960 represented a major improvement in neonatological clinical practice that led to improved newborn survival [1].
Respiratory disease is very frequently diagnosed in the perinatal period, especially in premature newborns with gestational ages (GA) under 32 weeks, the majority of which require long-term MV until the maturation of their respiratory system [2]. Reduced GA, low birth weight (BW), and increased MV duration lead to complications such as nosocomial infections, bronchopulmonary dysplasia (BPD), brain disorders, and retinopathy of prematurity (ROP), all leading to major long-term sequelae [3].
Synchronized intermittent positive pressure ventilation (SIPPV) and synchronized intermittent mandatory ventilation (SIMV) are among the most common forms of invasive MV [4]. MV can cause cerebral lesions via a local inflammatory process, due to hemodynamic instability [4]. In newborns, inflammation and changes in cerebral circulation can be responsible for intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), and hypoxic-ischemic encephalopathy development [5].
Calculating the adequate amount of oxygen is a very delicate issue, as both very high and very low levels of administered oxygen can be problematic [6]. Accordingly, an increase in the fraction of inspired oxygen (FiO2) can lead to hyperoxia in certain cases, causing cerebral vasoconstriction and retinal vasculature inhibition in premature newborns [6].
Moreover, MV has been demonstrated to be associated with various pathologies, including ROP, BPD, IVH, and PVL [7,8].
In recent years, nasal continuous positive airway pressure (nCPAP) therapy has become a widely-used alternative to MV in treating respiratory distress syndrome (RDS), with well-proven efficacy and relatively fewer associated risks compared with invasive MV therapy [9,10].
High-flow nasal cannula (HFNC) treatment has been reported to have similar positive end-expiratory pressure (PEEP) to nCPAP, with no significant differences found between the pulmonary mechanisms and gas exchanges [11]. Although HFNC therapy has been increasingly employed in recent years as an alternative respiratory support therapy in premature newborns with RDS [12], the differences between HFNC and nCPAP therapy in regard to ROP incidence have not been as well studied.
ROP, which was originally named retrolental fibroplasia, is a proliferative retinal blood vessel disease that can lead to visual impairment of varying severity [13,14]. It is characterized by abnormal growth of blood vessels in the retina of premature newborns, especially those undergoing oxygen therapy [15], and with BW <1500 g, in which case the incidence rate is approximately 60% [7]. It is a multifactorial disease that is very common in premature newborns, with various perinatal factors and interventions having been associated with an increased risk of developing ROP, including MV, oxygen therapy, red blood cell (RBC) transfusions, RDS, BPD, anemia, IVH, necrotizing enterocolitis, sepsis, and multiple pregnancies [7,16].
Retinal vascularization starts from intrauterine life and continues until term birth. In the case of premature newborns, the retinal angiogenesis process is interrupted, leading to an incompletely vascularized retina [17]. Intrauterine hypoxia due to increased metabolic needs during retinal neuron maturation has a strong impact on retinal vascularization, mediated by vascular endothelial growth factor (VEGF). In the first phase, hyperoxia reduces VEGF expression and inhibits normal vascularization, while in the second phase, hypoxia leads to an increase in VEGF expression and abnormal vascular proliferation [17]. Insulin-like growth factor 1 (IGF-1) is another important factor present in the angiogenesis process and leads to maximal stimulation of VEGF expression for retinal vascular development [18]. Decreased IGF-1 levels in premature newborns inhibit normal vascularization, and increased exposure to oxygen suppresses VEGF expression, therefore contributing to the inhibition of vascular development. As newborns age, the developing non-vascularized retina becomes hypoxic and the IGF-1 level slowly rises, enabling VEGF to stimulate an abnormal vascularization at the demarcation line between the vascular and avascular retina [19].
Unlike MV, which has been widely documented to be an ROP risk factor [7,8,16], nCPAP has been found to reduce the risk of retinal disease [20]; however, there are scarce data in the literature in regard to the relationship between nCPAP and ROP. A recent report has shown there is no statistically significant difference in terms of ROP risk between nCPAP and HFNC [21].
Moreover, the WHO “Recommendations on Interventions to Improve Preterm Birth Outcomes” guidelines specify that there is no significant relationship between ventilation using low concentrations of oxygen and ROP, highlighting the advantages of low oxygen concentration ventilation therapies [22].
Currently, in Romania, the national guide for screening and treatment of ROP, developed by the Ministry of Public Health and the Romanian Neonatology Association, is in the process of being finalized. Screening for ROP is carried out according to a national program for screening and treatment of ROP, established in 2002 at the Alessandrescu-Rusescu Institute for Mother and Child Health, Bucharest, by the Ministry of Public Health. Since 2004, this program has been implemented in other centers in the country. The ROP centers were established in level-3 maternity hospitals, to which, according to the legislation on the regionalization of maternal and child care, level-2 and level-1 maternity hospitals were attached [23].
The literature is unclear which of the 3 types of ventilation therapies confers the highest risk for developing ROP due to the lack of direct comparisons. When assessing which intervention has the highest benefit/risk ratio, clinicians need to have a better understanding of the risks associated with each intervention, including the risk of ROP.
The present study aimed to compare 3 different types of ventilation treatment, namely MV, nCPAP, and HFNC, as well as various perinatal characteristics, in preterm newborns <32 weeks GA, and the incidence of ROP at 21 days of life and later.
Material and Methods
ETHICS STATEMENT:
The study was conducted according to the guidelines of the Declaration of Helsinki and has received approval from the “Victor Babes” University of Medicine and Pharmacy Timisoara Ethical Committee for Scientific Research, namely approval no. 31/28 SEP 2018, as well as approval from the “Louis Ţurcanu” Emergency Hospital for Children Timisoara Ethical Committee for Scientific Research and Development (number 124/29 NOV 2023). Informed consent was obtained from parents or legal guardians for all patients admitted to the Neonatology and Preterm Infants Department.
STUDY DESIGN:
This study is a non-randomized controlled trial that consists of 3 intervention groups, namely MV, nCPAP, and HFNC, and a no-ventilation group. Given the nature of the experiment, randomization could not be performed due to ethical issues and the interventions were performed on an as-needed basis. The aim of this study is to investigate the relationship between different intervention groups and the incidence of ROP starting at 21 days of age by first performing univariate logistic regression, followed by multivariate logistic regression to account for all possible covariates in the given dataset.
STUDY POPULATION:
The 130 preterm newborns included in this study were hospitalized at the Premature Neonatology Clinic within the “Louis Ţurcanu” Emergency Hospital for Children in Timisoara, Romania between January 2019 and December 2020. The inclusion criteria were: premature newborns with GA under 32 weeks and BW under 2500 g and newborns that required MV and were admitted to the Intensive Neonatal Care section in the first week of life. The exclusion criteria were: premature newborns with cardiovascular, cerebral, or eye malformations; newborns who required MV and were admitted to the Intensive Neonatal Care section after the first week of life; and newborns deceased before 3 weeks of age.
PERINATAL MEASUREMENTS:
GA was determined by the last menstrual period. BW and Apgar scores were measured in the initial 5 postnatal minutes. The complete blood count was also performed on the first day of life by collecting 1 mL of peripheral venous blood and storing it in ethylenediaminetetraacetic acid (EDTA) disodium salt dihydrate, with follow-up measurements of hemoglobin (Hb), lactate dehydrogenase, and RBC levels being performed every 7 days of life or earlier depending on clinical circumstances.
RESPIRATORY SUPPORT:
In this study, 130 preterm newborns were included. The studied groups consisted of 54 newborns that underwent MV, 63 that underwent nCPAP, and 23 that underwent HFNC therapy, either alone or in combination with one of the other previously mentioned ventilation therapies. MV, either SIPPV or SIMV, was performed using either a Leoni Plus device (Löwenstein Medical SE & Co. KG, Bad Ems, Germany) or a Bellavista™ neo Ventilator device (Vyaire Medical Inc, Chicago, USA) with a peak inspiratory pressure of 18–20 cmH2O, a PEEP of 5–7 cmH2O, a respiratory rate of 30–40 breaths/min, and a fraction of inspired oxygen (FiO2) level of 0.4–0.6 [24,25]. NCPAP therapy was performed using either a Leoni Plus device (Löwenstein Medical) or a Bellavista™ neo Ventilator device (Vyaire Medical) with a PEEP of 5 cmH2O, while FiO2 was kept at the lowest possible value that permitted a 90–95% target oxygen saturation. HFNC therapy, namely Optiflow™ Nasal High-Flow therapy (Paykel Healthcare, Auckland, New Zealand), was administered at an air flux of 2–4 L/min and an FiO2 level of 0.3–0.4 [26].
OUTCOME DETERMINATION:
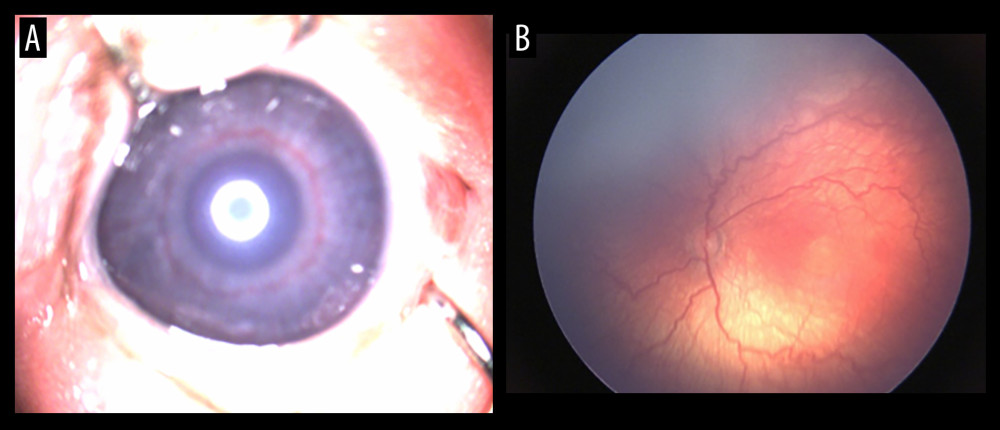
All newborns were evaluated by an experienced ophthalmologist using indirect ophthalmoscopy. The first examination was done 4 weeks after birth or at 30–31 weeks postmenstrual age. At 40 minutes before the examination, the newborn was instilled with mydriatic drops to dilate the pupil (tropicamide 0.5% or phenylephrine 2.5%), 1 drop each in both eyes, 2–3 times, at an interval of approximately 5 minutes. The blepharostat and the scleral indenter were used after administration of ocular local anesthetic. Indirect ophthalmoscopy was performed using the Vantage Plus indirect ophthalmoscope (Keeler, Windsor, UK) 1 hour after feeding [27]. A 28 D condensing lens was used, which offers the advantage of a wider viewing area. RetCam Shuttle (Clarity Medical Systems, Pleasanton, CA, USA) was used for the digital acquisition of images in the screening and management of ROP [28]. The rhythmicity of the examinations was dictated by the severity of the disease and varied between a few days and a few weeks. The results of the examination were recorded in the observation sheet of each patient. ROP was classified as per “The International Classification of Retinopathy of Prematurity” [29]. Figure 1A, 1B exemplifies a vascular engorgement of the iris, as well as vascular changes in the posterior pole, such as arteriolar tortuosity and venular dilatation.
STATISTICAL ANALYSIS:
Statistical analysis was performed using 2-tailed
Results
POPULATION CHARACTERISTICS:
Out of 130 premature newborns enrolled in this study, 54 underwent MV therapy, 63 underwent nCPAP therapy, and 23 underwent HFNC therapy, either alone or in combination with one of the other previously mentioned ventilation therapies. The incidence rate of ROP in the MV, nCPAP, and HFNC groups was 64.81% (n=35), 73.02% (n=46), and 60.87% (n=14). ROP was not diagnosed in any of the 13 newborns who did not undergo respiratory support. Baseline differences between the groups were analyzed using
UNIVARIATE ANALYSIS:
Univariate (one independent factor at a time) binomial (dichotomized dependent variable) analysis was performed following the above-mentioned variable classification. As shown in Table 1, multiple factors were associated with an increased risk of ROP, including protective factors, namely GA, BW, 1- and 5-minute Apgar scores, and day 1 Hb, HCT, and RBC levels, and risk factors, namely male sex, transfusions, and nCPAP therapy.
Day 1 RBC count was the strongest protective factor, with an OR of 0.472 (95% CI, 0.258–0.863;
RBC transfusions represented the strongest risk factor associated with ROP at 21 days of life or later, with an OR of 5.250 (95% CI, 2.465–11.182;
MULTIVARIATE ANALYSIS:
Factors with a statistically significant correlation to ROP following univariate analysis were then analyzed using multivariate binomial logistic regression analysis, and the adjusted ORs were calculated. The Omnibus Tests of Model Coefficients were used to examine whether or not there is a significant impact of the included factors on the prediction of ROP incidence. A
The results of the multivariate logistic regression analysis are displayed in Table 2. Dissimilar to the results of the univariate logistic regression analysis, the only 2 factors that remained statistically significant were BW (P<0.05) and nCPAP (P<0.001). BW was demonstrated to be a protective factor against developing ROP, with an adjusted odds ratio (AOR) of 0.998 (95% CI, 0.996–0.999); however, the effect size was negligible. Surprisingly, transfusions, which constituted the strongest significant risk factor at a univariate level, were no longer statistically significant when adjusting for covariates, with an AOR of 2.413 (95% CI, 0.874–6.667; P=0.089). The sole significant risk factor following multivariate analysis was nCPAP, which was shown to increase the risk of neonates developing ROP over 7-fold, having an AOR of 7.264, (95% CI, 2.622–20.120). However, the wide confidence interval stems from a relatively small sample size and, therefore, warrants further research involving larger data sets.
Discussion
Our results show that, at a univariate level, increased GA, BW, 1- and 5-minute Apgar scores, and day-1 Hb, HCT, and RBC levels are associated with reduced risk of ROP, while male sex, transfusions, and nCPAP therapy are associated with an increased risk of ROP. However, when accounting for all the possible covariates included in the data, BW was the only remaining ROP protective factor, while nCPAP was the only remaining ROP risk factor. MV and HFNC were not significantly associated with ROP at either the univariate or multivariate analysis level.
Increased GA has been shown in numerous reports to be a significant protective factor, especially for newborns aged 24–26 weeks [3,7,30–40]. This coincides with our results at a univariate level; however, our results did not indicate a significant association between GA and ROP when adjusting for covariates (AOR=1.169; 95% CI, 0.874–1.564;
Increased BW has also been previously identified as a protective factor, albeit a weaker one than GA, throughout the literature [3,16,30–34]. BW was found to be a protective factor in our study at both a univariate level and after adjusting for covariates; however, as expected, the effect size was minor (AOR=0.998; 95% CI, 0.996–0.999;
The univariate analysis results showed both 1- and 5-minute Apgar scores are a significant predictive factor for ROP, with increased scores being associated with reduced ROP incidence. Similar results have been previously showcased in the literature [41,42]. Surprisingly, in our study, neither the 1- nor 5-minute Apgar score was significantly associated with ROP when adjusting for covariates, with an AOR of 0.775 (95% CI, 0.500–1.201;
Complete blood count parameters, such as Hb level, HCT level, and RBC count, have been previously demonstrated to be inversely correlated with ROP incidence [43]. Accordingly, all 3 parameters were found to be significant protective factors against ROP at a univariate level. Following multivariate analysis, none of the 3 blood count parameters remained significantly associated with ROP, having AORs of 0.764 (95% CI, 0.373–1.565;
Interestingly, sex was not found to be significantly correlated with ROP in previous studies [36,38]. However, male sex was significantly associated with increased ROP risk in our study following univariate analysis. This is especially unexpected, as a similar recent study found that ROP risk was actually increased for female sex [40]. As expected, male sex was no longer associated with ROP after adjusting for covariates, having an AOR of 2.646 (95% CI, 0.955–7.326;
RBC transfusions, which have been previously associated with ROP [7,8], have also been shown to considerably increase the risk of developing anemia of prematurity (AOP) at 21 days of age or later, by more than 5-fold, when studied at a univariate level. Following adjustment for potential covariates, interestingly, transfusions were no longer significantly associated with AOP, having an AOR of 2.413 (95% CI, 0.874–6.667;
Most surprisingly, MV, a well-known ROP risk factor [7,8,16], was not significantly associated with ROP at a univariate level, having an OR of 1.942 (95% CI, 0.948–3.978;
HFNC has also been associated with ROP across multiple studies [44,45]. However, our results indicated no significant relationship between HFNC and ROP at a univariate level, with HFNC having an OR of 1.314 (95% CI, 0.524–3.297;
The sole remaining risk factor following multivariate regression analysis, nCPAP, which in our study had an AOR of 7.264 (95% CI, 2.622–20.120;
Therefore, the results of our study offer a novel perspective on the relationship between ventilation and ROP. While in the literature, MV and HFNC are the therapies most commonly associated with ROP [7,8,14,44,45], in our study it was the less reputed ROP risk factor, namely nCPAP, that had the strongest correlation with ROP incidence, while MV and HFNC were not significant risk factors at any level of analysis. This is especially surprising following the results of a recent systematic review, in which the pooled results of a meta-analysis of 3 studies comparing nCPAP and HFNC in terms of risk of ROP were not statistically significant [46].
The inability to perform randomization due to ethical concerns represents the main limitation of this study, followed by occasional overlapping ventilation treatments, which had to be administered due to clinical circumstances. Another major limitation is the small sample size of this study which precluded various tests. The main issues that stemmed from this limitation were the lack of ROP cases in the no-intervention group, precluding a direct comparison with intervention groups, and the relatively few cases of Stage 1, 3, and 4 ROP, which precluded a more detailed analysis using specific ROP stages as the outcomes instead of any-stage ROP. The relatively small number of perinatal factors available for this study also posed a limitation in compensating for even more possible covariates when calculating adjusted ORs. Moreover, there are possible limitations associated with the RetCam device used to diagnose ROP, namely low resolution and limitation in capturing dark fundi [47].
Conclusions
Following a multivariate regression analysis, only BW and nCPAP therapy were significantly associated with ROP. The results indicated that BW was a weak protective factor against ROP, nCPAP was a strong ROP risk factor, while MV and HFNC were not associated with ROP.
Tables
Table 1. Univariate binary logistic regression analysis results showing the associations between different interventions or perinatal characteristics and ROP at 21 days of life.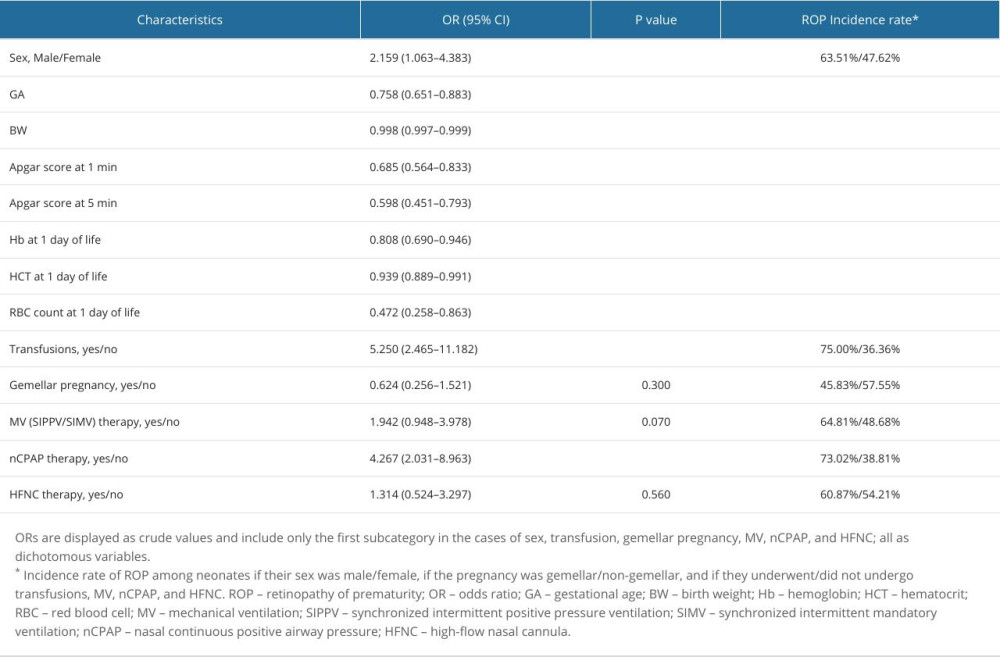 Table 2. Multivariate binary logistic regression analysis results showing the associations between different interventions or perinatal characteristics and ROP.
Table 2. Multivariate binary logistic regression analysis results showing the associations between different interventions or perinatal characteristics and ROP.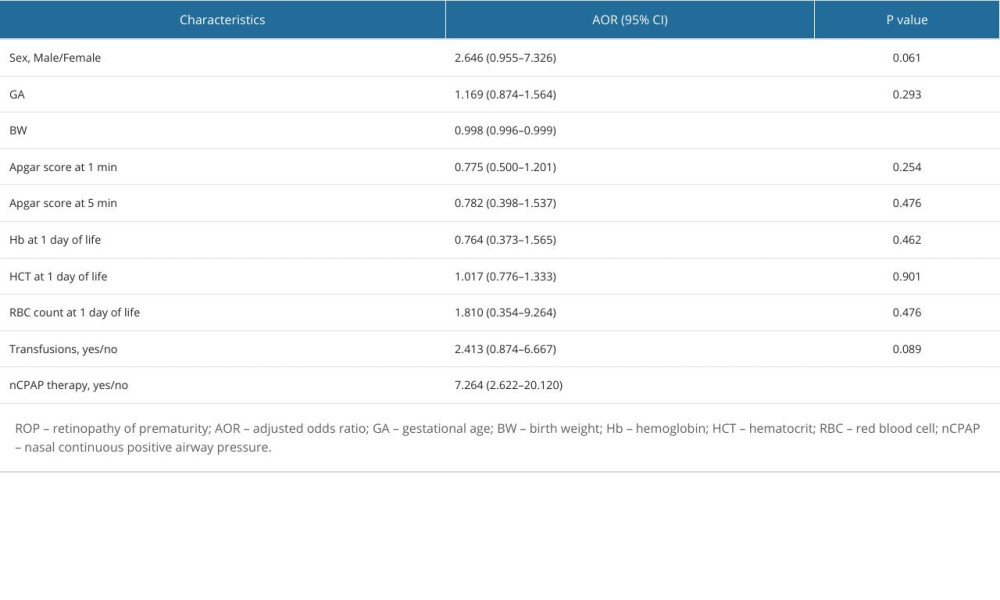
References
1. Lista G, Catoldi F, Fontana P, Lung inflammation in preterm infants with respiratory distress syndrome: Effects of ventilation with different tidal volumes: Pediatr Pulmonol, 2006; 41(4); 357-63
2. Yue G, Wang J, Li H, Risk factors of mechanical ventilation in premature infants during hospitalization: Ther Clin Risk Manag, 2021; 17; 777-87
3. Booth EA, Dukatz C, Sood BG, Wider M, Near-infrared spectroscopy monitoring of cerebral oxygen during assisted ventilation: Surg Neurol Int, 2011; 2; 65
4. Chakkarapani AA, Adappa R, Mohammad Ali SK, “Current concepts in assisted mechanical ventilation in the neonate” – Part 2: Understanding various modes of mechanical ventilation and recommendations for individualized disease-based approach in neonates: Int J Pediatr Adolesc Med, 2020; 7(4); 201-8
5. Cannavò L, Rulli I, Falsaperla R, Ventilation, oxidative stress and risk of brain injury in preterm newborn: Ital J Pediatr, 2020; 46(1); 100
6. Giliberti P, De Leonibus C, Chello G, Near-infrared spectroscopy in Neonatal Intensive Care Unit: Do we make our life more difficult?: J Pediatr Neonat Individual Med, 2013; 2(2); e020223
7. Chang JW, Risk factor analysis for the development and progression of retinopathy of prematurity: PLoS One, 2019; 14(7); e0219934
8. Slidsborg C, Jensen A, Forman JL, Neonatal risk factors for treatment-demanding retinopathy of prematurity: A Danish National Study: Ophthalmology, 2016; 123(4); 796-803
9. Mahmoud RA, Schmalisch G, Oswal A, Non-invasive ventilatory support in neonates: An evidence-based update: Paediatr Respir Rev, 2022; 44; 11-18
10. Moreel L, Proesmans M, High flow nasal cannula as respiratory support in treating infant bronchiolitis: A systematic review: Eur J Pediatr, 2020; 179(5); 711-18
11. Lavizzari A, Colnaghi M, Ciuffini F, Heated, humidified high-flow nasal cannula vs nasal continuous positive airway pressure for respiratory distress syndrome of prematurity: A randomized clinical noninferiority trial: JAMA Pediatr, 2016 [Online ahead of print]
12. Manley BJ, Owen LS, High-flow nasal cannula: Mechanisms, evidence and recommendations: Semin Fetal Neonatal Med, 2016; 21(3); 139-45
13. Malik MA, Shukla S, Azad SV, Vascular endothelial growth factor (VEGF-634G/C) polymorphism and retinopathy of prematurity: A meta-analysis: Saudi J Ophthalmol, 2014; 8(4); 299-303
14. Hellström A, Smith LE, Dammann O, Retinopathy of prematurity: Lancet, 2013; 382(9902); 1445-57
15. Brown AC, Nwanyanwu K, Retinopathy of prematurity: StatPearls, 2023 Available from: https://www.ncbi.nlm.nih.gov/books/NBK562319/
16. Borţea CI, Stoica F, Boia M, Risk factors associated with retinopathy of prematurity in very and extremely preterm infants: Medicina (Kaunas), 2021; 57(5); 420
17. Kwinta P, Bik-Multanowski M, Mitkowska Z, The clinical role of vascular endothelial growth factor (VEGF) system in the pathogenesis of retinopathy of prematurity: Graefes Arch Clin Exp Ophthalmol, 2008; 246(10); 1467
18. Smith LE, IGF-1 and retinopathy of prematurity in the preterm infant: Biol Neonate, 2005; 88(3); 237-44
19. Liegl R, Löfqvist C, Hellström A, IGF-1 in retinopathy of prematurity, a CNS neurovascular disease: Early Hum Dev, 2016; 102; 13-19
20. García-Sánchez A, Villalaín I, Jaureguizar A, CPAP effect on progression of retinal disease in patients with sleep apnea and non-proliferative diabetic retinopathy: A randomized clinical trial: Ann Am Thorac Soc, 2024; 21(1); 102-13
21. Chen J, Lin Y, Du L, The comparison of HHHFNC and NCPAP in extremely low-birth-weight preterm infants after extubation: A single-center randomized controlled trial: Front Pediatr, 2020; 8; 250
22. : WHO Recommendations on Interventions to Improve Preterm Birth Outcomes, 2015, Geneva, World Health Organization Available from: https://www.ncbi.nlm.nih.gov/books/NBK321160/
23. : National Recovery and Resilience Plan, 2022, Romania, Ministry of Public Health Available from: [in Romanian]https://www.ms.ro/media/documents/Ghidul-Beneficiarului_8ePMsWj.pdf
24. Leoni plus: Lowenstein Medical Available from:https://hul.de/uk/produkt/leoni-plus-3/
25. : bellavista™ neo Ventilator, Vyaire Medical Available from:https://intl.vyaire.com/products/bellavista-neo-ventilator/
26. : Optiflow™, Paykel Healthcare Available from:https://www.fphcare.com/us/hospital/adult-respiratory/optiflow/
27. : Vantage Plus Indirect Ophthalmoscope, Keeler Available from: https://www.keeler.co.uk/vantage-plus-led-indirect-ophthalmoscope.html/
28. RetCam Shuttle, Clarity Medical Systems Available from:https://www.yumpu.com/en/document/view/18702942/the-retcam-shuttle-clarity-medical-systems/
29. International Committee for the Classification of Retinopathy of Prematurity, The International Classification of Retinopathy of Prematurity revisited: Arch Ophthalmol, 2005; 123(7); 991-99
30. Wang ZH, Li YY, Liu ZM, Birth weight and gestational age on retinopathy of prematurity in discordant twins in China: Int J Ophthalmol, 2014; 7(4); 663-67
31. Wu T, Rao R, Gu H, Retinopathy of prematurity: Risk stratification by gestational age: J Perinatol, 2023; 43(6); 694-701
32. Freitas AM, Mörschbächer R, Thorell MR, Incidence and risk factors for retinopathy of prematurity: A retrospective cohort study: Int J Retina Vitreous, 2018; 4; 20
33. Friling R, Axer-Siegel R, Hersocovici Z, Retinopathy of prematurity in assisted versus natural conception and singleton versus multiple births: Ophthalmology, 2007; 114(2); 321-24
34. Palmer EA, Flynn JT, Hardy RJ, Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group: Ophthalmology, 1991; 98(11); 1628-40
35. Austeng D, Källen KB, Ewald UW, Incidence of retinopathy of prematurity in infants born before 27 weeks’ gestation in Sweden: Arch Ophthalmol, 2009; 127(10); 1315-19
36. Hussain N, Clive J, Bhandari V, Current incidence of retinopathy of prematurity, 1989–1997: Pediatrics, 1999; 104(3); e26
37. Ying GS, VanderVeen D, Daniel ETelemedicine Approaches to Evaluating Acute-Phase Retinopathy of Prematurity Cooperative Group, Risk score for predicting treatment-requiring retinopathy of prematurity (ROP) in the telemedicine approaches to evaluating acute-phase ROP study: Ophthalmology, 2016; 123(10); 2176-82
38. Yang MB, Rao S, Copenhagen DR, Length of day during early gestation as a predictor of risk for severe retinopathy of prematurity: Ophthalmology, 2013; 120(12); 2706-13
39. Holmström G, Broberger U, Thomassen P, Neonatal risk factors for retinopathy of prematurity – a population-based study: Acta Ophthalmol Scand, 1998; 76(2); 204-7
40. Lin YW, Chen SN, Muo CH, Risk of retinopathy of prematurity in preterm births with respiratory distress syndrome: A population-based cohort study in Taiwan: Int J Gen Med, 2022; 15; 2149-62
41. Alajbegovic-Halimic J, Zvizdic D, Alimanovic-Halilovic E, Risk factors for retinopathy of prematurity in premature born children: Med Arch, 2015; 69(6); 409-13
42. Marinov VG, Koleva-Georgieva DN, Sivkova NP, The 5-minute Apgar score as a prognostic factor for development and progression of retinopathy of prematurity: Folia Med (Plovdiv), 2017; 59(1); 78-83
43. Akyüz Ünsal Aİ, Key Ö, Güler D, Can complete blood count parameters predict retinopathy of prematurity?: Turk J Ophthalmol, 2020; 50(2); 87-93
44. Healy LI, Corcoran P, Murphy BP, High-flow nasal cannulae, bronchopulmonary dysplasia and retinopathy of prematurity: Ir Med J, 2019; 112(8); 985
45. Hoffman SB, Terrell N, Driscoll CH, Impact of high-flow nasal cannula use on neonatal respiratory support patterns and length of stay: Respir Care, 2016; 61(10); 1299-304
46. Luo K, Huang Y, Xiong T, High-flow nasal cannula versus continuous positive airway pressure in primary respiratory support for preterm infants: A systematic review and meta-analysis: Front Pediatr, 2022; 10; 980024
47. Valikodath N, Cole E, Chiang MF, Imaging in retinopathy of prematurity: Asia Pac J Ophthalmol (Phila), 2019; 8(2); 178-86
Tables
 Table 1. Univariate binary logistic regression analysis results showing the associations between different interventions or perinatal characteristics and ROP at 21 days of life.
Table 1. Univariate binary logistic regression analysis results showing the associations between different interventions or perinatal characteristics and ROP at 21 days of life. Table 2. Multivariate binary logistic regression analysis results showing the associations between different interventions or perinatal characteristics and ROP.
Table 2. Multivariate binary logistic regression analysis results showing the associations between different interventions or perinatal characteristics and ROP. Table 1. Univariate binary logistic regression analysis results showing the associations between different interventions or perinatal characteristics and ROP at 21 days of life.
Table 1. Univariate binary logistic regression analysis results showing the associations between different interventions or perinatal characteristics and ROP at 21 days of life. Table 2. Multivariate binary logistic regression analysis results showing the associations between different interventions or perinatal characteristics and ROP.
Table 2. Multivariate binary logistic regression analysis results showing the associations between different interventions or perinatal characteristics and ROP. In Press
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952