14 May 2024: Clinical Research
Correlation between Thalamocortical Tract and Default Mode Network with Consciousness Levels in Hypoxic-Ischemic Brain Injury Patients: A Comparative Study Using the Coma Recovery Scale-Revised
Sung Ho JangDOI: 10.12659/MSM.943802
Med Sci Monit 2024; 30:e943802
Abstract
BACKGROUND: The thalamocortical tract (TCT) links nerve fibers between the thalamus and cerebral cortex, relaying motor/sensory information. The default mode network (DMN) comprises bilateral, symmetrical, isolated cortical regions of the lateral and medial parietal and temporal brain cortex. The Coma Recovery Scale-Revised (CRS-R) is a standardized neurobehavioral assessment of disorders of consciousness (DOC). In the present study, 31 patients with hypoxic-ischemic brain injury (HI-BI) were compared for changes in the TCT and DMN with consciousness levels assessed using the CRS-R.
MATERIAL AND METHODS: In this retrospective study, 31 consecutive patients with HI-BI (17 DOC,14 non-DOC) and 17 age- and sex-matched normal control subjects were recruited. Magnetic resonance imaging was used to diagnose HI-BI, and the CRS-R was used to evaluate consciousness levels at the time of diffusion tensor imaging (DTI). The fractional anisotropy (FA) values and tract volumes (TV) of the TCT and DMN were compared.
RESULTS: In patients with DOC, the FA values and TV of both the TCT and DMN were significantly lower compared to those of patients without DOC and the control subjects (p<0.05). When comparing the non-DOC and control groups, the TV of the TCT and DMN were significantly lower in the non-DOC group (p<0.05). Moreover, the CRS-R score had strong positive correlations with the TV of the TCT (r=0.501, p<0.05), FA of the DMN (r=0.532, p<0.05), and TV of the DMN (r=0.501, p<0.05) in the DOC group.
CONCLUSIONS: This study suggests that both the TCT and DMN exhibit strong correlations with consciousness levels in DOC patients with HI-BI.
Keywords: Hypoxia-Ischemia, Brain, Consciousness, Default Mode Network, diffusion tensor imaging
Introduction
Hypoxic-ischemic brain injury (HI-BI) is a severe consequence arising from cerebral oxygen deprivation, resulting in metabolic deficiencies [1]. It is frequently caused by cardiac arrest, carbon monoxide poisoning, and strangulation, among others [1]. Common sequela of HI-BI includes disorders of attention, memory impairments, altered processing speeds, and impaired consciousness, also referred to as disorders of consciousness (DOC) [2,3]. The incidence of hypoxic brain injury varies depending on the underlying cause, but it can have devastating effects on neurological function and overall quality of life [4]. Diagnosis of HI-BI typically involves a comprehensive evaluation of clinical symptoms, medical history, neurological examinations to assess cognitive and motor function, and neuroimaging studies such as MRI or CT scans [4]. Management of HI-BI often involves immediate resuscitation and restoration of oxygen supply to the brain through artificial ventilation or medication to support cardiovascular function [4]. Previous studies have reported that only 27% of coma patients who had suffered from HI-BI regained consciousness within 28 days, with over 60% succumbing and 9% persisting with impaired consciousness [1]. Furthermore, the potentially positive effects of acute interventions, such as that of therapeutic hypothermia, remain uncertain for patients with HI-BI [3]. Consequently, examining the neural correlates associated with impaired consciousness in DOC patients with HI-BI is clinically important for predicting prognosis and the development of neurorehabilitation guidelines.
Exploration of the various neurological circuits of consciousness control revealed the ascending reticular activating system (ARAS), default mode network (DMN), frontoparietal network, and the frontostriatal network to be key tracts and networks involved in the control of consciousness [5–13]. Notably, the thalamocortical tract (TCT), derived from thalamocortical radiations of the ARAS, and the DMN emerge as critical tracts involved in controlling consciousness [5–13]. Thalamocortical radiations, particularly those involving the intralaminar nuclei, contribute to the regulation of cortical arousal and consciousness, with disruptions in these pathways are directly correlated with the loss of consciousness observed in DOC patients [14]. The TCT, which connects the intralaminar thalamic nuclei (ILN) and the cerebral cortex, is known to be involved in awareness, specifically in providing the necessary arousal to bring about awareness [7,8,15]. Functionally, the TCT relays visual, auditory, somatosensory, motor, and other sensory modalities to the cerebral cortex, playing an important role in cortical arousal [14]. The DMN exhibits connectivity to several parts of the brain, namely, the precuneus/posterior cingulate cortex (PCC), medial prefrontal cortex (mPFC), bilateral temporo-parietal junctions, and the hippocampal formation [5,10]. These interconnected regions are consistently active when an individual is not engaged in any specific cognitive task, and become more active during periods of rest, introspection, and tasks that require internally focused attention [10]. Among these regions, the connections found between the precuneus/PCC and mPFC were recognized to be critical in consciousness and associated with self-reflection and self-awareness [5,9,16]. Dysfunction or alterations in the default mode network have been implicated in various psychiatric disorders, including schizophrenia, depression, and autism spectrum disorders [10].
Despite extensive use of varying neuroimaging techniques like diffusion tensor imaging (DTI), diffusion tensor tractography (DTT), resting-state functional magnetic resonance (rs-fMRI), and electroencephalography (EEG) for examining consciousness levels in patients with various brain pathologies such as HI-BI, stroke, traumatic brain injuries, and encephalitis, no studies to date have compared both the TCT and the DMN regarding their relationship with consciousness levels [17–23].
Among the mentioned neuroimaging techniques, DTT, a relatively novel technique, distinguished itself for its ability to quantitatively assess white matter integrity and three-dimensionally visualize and estimate the brain’s structural neural connectivity [24,25]. DTT is derived from DTI, which analyzes the anisotropic movement of water molecules in the white matter of the brain [26]. Several DTT-based studies have investigated the relationship between consciousness levels and the DMN or the TCT in HI-BI patients [27–30]. These studies revealed correlations between the connectivity of the TCT or the DMN with consciousness levels, assessed through the Coma Recovery Scale-Revised (CRS-R) and Glasgow Coma Scale (GCS), in patients with early-stage HI-BI [27–30]. In a study comparing the DMN to consciousness levels in patients with traumatic brain injury, a close correlation was found between consciousness levels and the mPFC to PCC tract as well as the mPFC to precuneus tract [9]. Another study comparing the TCT to consciousness levels in HI-BI patients determined a close relationship between the prefrontal area of the TCT and CRS-R scores [29]. However, there have not been any DTT-based studies to date, comparing the relationship of both the TCT and the DMN to consciousness levels in DOC patients with HI-BI. Therefore, this study of 31 patients with HI-BI aimed to compare changes in the TCT and the DMN and levels of consciousness assessed using the CRS-R.
In the present study, we hypothesized that the TCT and the DMN are closely associated with consciousness levels in patients with HI-BI. We utilized DTT to compare the relationship of the DMN and TCT with the consciousness levels of DOC patients with HI-BI.
Material and Methods
SUBJECTS:
This retrospective study, which received approval from the Institutional Review Board at Yeungnam University Hospital in Daegu, South Korea, adhered to protocols ensuring written informed consent was obtained from all participating patients for the publication of this paper. A total of 31 patients (19 male patients and 12 female patients; mean age of 49.42±13.16 years; age range of 18–76 years) with HI-BI and 17 age- and sex-matched normal, control subjects (mean age of 48.94±14.11 years, age range 24–77 years) with no history of HI-BI or neurologic/psychiatric disease were enrolled into this study. The inclusion parameters used for these 31 patients were as follows: (1) An apparent HI-BI history with bilateral lesions identified using MRI, resulting from events such as cardiac arrest, carbon monoxide intoxication, etc.; (2) Impaired consciousness (determined by a CRS-R score <23) at the onset of HI-BI; (3) DTI scanning taken at least one month after HI-BI onset; (4) The age range of the patient at the onset of HI-BI 18~80 years; (5) No previous history of trauma to the head or disease with neurologic/psychiatric origins.
CLINICAL EVALUATION:
In order to evaluate consciousness levels, the CRS-R was employed. The CRS-R is a standard neurobehavioral assessment scale that is used to evaluate consciousness levels of patients with DOC, consisting of 6 subscales (auditory, visual, motor, oromotor, communication, and arousal) [31]. Each subscale consists of specific items graded on a hierarchical scale, allowing for comprehensive evaluation of the patient’s levels of awareness and responsiveness [32]. The CRS-R score ranges between 0 and 23 and can distinguish a patient’s consciousness state (vegetative state, minimally conscious state, and/or emergence from a minimally conscious state) [31]. The sensitivity and specificity of the CRS-R score to detect consciousness levels and awareness were well established [33].
The patient group (31 patients) was divided into 2 subgroups based on the CRS-R scores determined at the time of DTI scanning: 17 patients were assigned to the disorder of consciousness (DOC) group (impaired consciousness; CRS-R score <23) and 14 patients in the non-DOC group (intact consciousness; CRS-R score=23). At the time of DTI scanning, the average CRS-R score for the DOC group was 7.29±3.42. Seventeen HI-BI patients with DOC (11 male patients and 6 female patients; mean age of 48.53±13.79 years; mean duration from HI-BI onset to DTI 6.78±8.35 months), 14 HI-BI patients without DOC (8 male patients and 6 female patients; mean age 50.50±12.78 years; mean duration to DTI 6.96±6.80 months), and 17 age- and sex-matched control subjects (11 males and 6 females; mean age 48.94±14.11 years) were selected for this study. There were no significant differences found in the distribution of age and gender among the groups, or in the duration of time from HI-BI onset to DTI imaging between the DOC and non-DOC groups (
DIFFUSION TENSOR IMAGING AND FIBER TRACKING:
DTI scanning was conducted at an average of 6.86±7.57 months after HI-BI onset using a 1.5T Philips Gyroscan Intera scanner (Hoffman-LaRoche, Best, The Netherlands) with a six-channel head coil. A single-shot, spin-echo planar imaging method was used. For each of the 32 noncollinear diffusion-sensitizing gradients, 67 contiguous slices were acquired parallel to the anterior commissure-posterior commissure line. The imaging parameters were as follows: acquisition matrix=96×96, reconstructed to matrix=128×128, field of view=221×221 mm2, repetition time=10,726 ms, echo time=76 ms, parallel imaging reduction factor (sensitivity encoding factor)=2, echo planar imaging factor=49, b=1000 s/mm2, number of excitations=1, and slice thickness=2.3 mm. The Oxford Centre for Functional Magnetic Resonance Imaging of Brain (FMRIB) Software Library (FSL:
The TCT was reconstructed by selecting fibers passing through a seed region of interest (ROI) on the ILN between the anterior and posterior commissures at the level of the inter-commissural [15]. The fibers stretching out below the ILN or extending into the contralateral hemisphere were excluded. The DMN was reconstructed by selecting the fibers passing from a seed ROI covering the PCC and the precuneus in the coronal view [9]. Target ROIs were manually located on the anterior cingulate cortex in the coronal view (Brodmann’s areas 24, 32, and 33) and on the mPFC in the coronal view (Brodmann’s areas 14, 24, 25, and 32) [9,15]. The superior boundary of the target ROI in the mPFC was defined by the cingulate sulcus, the medial boundary was marked by the midline separating the right and left hemispheres, and the lateral boundary was a line set at a distance of 11.25 mm lateral from the midline [9]. The fibers passing through the contralateral hemisphere were excluded. Of the 5000 samples generated from the seed voxel, results for positive contacts were visualized at a minimum threshold of 10 and 2 streamlines through each voxel for the TCT and the DMN, respectively. The average value of the DTT parameters (fractional anisotropy [FA] value and tract volume [TV]) from the bilateral hemispheres were measured for the TCT and DMN (Figure 1).
STATISTICAL ANALYSIS:
All statistical analyses were performed using SPSS 21.0 (SPSS, Chicago, Illinois, USA). A chi-squared test was used to assess the differences in sex populations among the groups, and an independent t-test was used to evaluate the significant differences in the duration from HI-BI onset to DTI imaging. One-way analysis of variance (ANOVA) was used to assess the significant differences in age populations and used to determine the differences in the DTT parameters of the DMN and TCT among the groups. The Bonferroni post hoc test was conducted to determine the significant differences between the groups. The Spearman correlation coefficient was used to determine the correlations between the DTT parameters of the DOC group and their CRS-R scores. A correlation coefficient greater than 0.50 represented a strong correlation, between 0.30 and 0.49 represented a moderate correlation, and between 0.10 and 0.29 represented a weak correlation [34]. A result was deemed significant if the p-value was less than 0.05.
Results
COMPARISONS OF THE DTT PARAMETERS:
The results of the comparisons of the DTT parameters among the groups are summarized in Table 1. When the non-DOC and control groups were compared, the TV value of the TCT and of the DMN were significantly lower in the non-DOC group (p<0.05), whereas the FA of the TCT and of the DMN showed no significant differences between the groups (p>0.05). The FA values and TV of the TCT and DMN for the DOC group were significantly lower than those of the non-DOC group and control group (p<0.05).
CORRELATIONS BETWEEN CRS-R SCORES AND DTT PARAMETERS:
The correlation between the CRS-R scores and the DTT parameters of the TCT and DMN of the DOC group are represented in Table 2. The CRS-R score had strong positive correlations with the TV of the TCT (r=0.501, p<0.05), the FA of the DMN (r=0.532, p<0.05), and the TV of the DMN (r=0.501, p<0.05). No significant correlations were seen between the FA value of the TCT and the CRS-R score (p>0.05).
Discussion
In this DTT-based study, we compared the relationship of the TCT and the DMN with the consciousness level in patients with HI-BI. The results are summarized as follows: (1) the FA values and TV of the TCT and DMN were lower in the DOC group compared to both the non-DOC and control groups, and the TV of the TCT and the DMN were lower in the non-DOC group compared to the control group, and (2) strong positive correlations were observed between the CRS-R score and the TV of the TCT, FA of the DMN, and TV of the DMN in the DOC group.
The FA value is the most widely used DTT parameter and represents the motional anisotropy of water molecules derived from the integrity of white matter microstructures such as axons, myelin, and microtubules [24,25]. Conversely, the TV indicates the number of voxels within a neural tract, alluding to the total number of neural fibers [24,25]. Thus, the low FA values and low TV of the TCT and of the DMN in the DOC and non-DOC groups, when compared to the control group, indicate decreased microstructure integrity and fewer neural fibers constituting the tract, suggesting neural injury in the patient groups. When comparing the DOC group and non-DOC groups, the significant reduction in the FA values and TV of the DMN and TCT for the DOC group suggests a greater severity of neural injury in the DOC group than in the non-DOC group. On the other hand, a strong positive correlation between the CRS-R score and the TV of the TCT, the FA of the DMN, and the TV of the DMN was found, suggesting that the number of neural fibers composing the TCT and the DMN are closely related to consciousness levels.
The lack of blood flow to the brain during hypoxic ischemia and the consequent low levels of glucose lead to a decrease in adenosine triphosphate (ATP) production which causes an ion imbalance [4,35–37]. Neuronal cells affected by this imbalance accumulate sodium and resulting in the release of the neurotransmitter glutamate, activating several lipases, proteases, and nucleases that break down and cause damage to neuronal tissue throughout the brain [4,36,37]. The severity of the neuronal injury, however, is not the same throughout, and several studies on various animal models found that regions of the thalamus, basal ganglia, hippocampus, and neocortex sustained the most damage after injury [4,35–44]. Since the thalamus is vulnerable to HI-BI, the resulting strong correlation between the TCT, which originates from the ILN of the thalamus, and consciousness levels could be expected. In addition, tractography analysis revealed that the thalamus and basal forebrain have high centrality in the DMN, with connectivity seen between the thalamus and the medial and ventrolateral prefrontal regions, as well as between the basal forebrain and the mPFC, posterior cingulate region, retrosplenial cortex, and hippocampus [45]. Thus, both the TCT and the DMN could be considered potential neuronal indicators closely related to consciousness levels in patients with HI-BI.
Several studies have been conducted on the relationship between the TCT or the DMN and consciousness levels in patients with HI-BI [26–29]. In 2019, Stafford et al identified a significant decrease in the FA value of the TCT in patients with DOC compared to healthy controls and correlations between the TCT and CRS-R scores in 15 patients with brain pathologies (HI-BI: 7 patients, traumatic brain injury: 7 patients, and other: one patient) [30]. In 2022, Jang and Choi found a positive correlation between the GCS and CRS-R scores with the FA value and the TV of the mPFC’s structural neural connectivity in 23 DOC patients with HI-BI [27]. In another study, Jang and Choi (2022) found significant decreases in the FA value and the TV of the TCT in 14 DOC patients with HI-BI [28]. Recently, Jang and Choi (2023) analyzed the 5 subparts of the TCT (the prefrontal, premotor, primary motor, somatosensory, and posterior parietal parts) and found a moderate positive correlation between the CRS-R score and the TV of the prefrontal part in 17 DOC patients with HI-BI [29]. As a result, to the best of the authors’ knowledge, this study is the first to compare the relationships of both the TCT and DMN to consciousness levels in patients with HI-BI or with any brain pathology.
However, several limitations of this study should be considered. First, DTT reconstruction is operator-dependent and based on the fiber tracking technique used. False-positive and false-negative results can occur due to crossing fibers or the partial volume effect. In addition, due to the retrospective nature of the study, we could not conduct additional analyses using neuroimaging methods such as fMRI and EEG to cross-validate the results. Second, since the introduction of the DTT technique, only the DMN and the TCT of the ARAS have been analyzed to identify the neural injury or neural correlation to the clinical evaluation associated with consciousness in DOC patients. In our study, we only reconstructed the TCT and the DMN among the various neural networks related to consciousness, such as the lower ARAS, frontoparietal network, and frontostriatal network [5–13,17,22,27–30]. Third, since the study was performed retrospectively, only the CRS-R was used to evaluate consciousness levels without conducting any cross-validation for the accuracy of the evaluation in the patients with HI-BI. Fourth, only 48 subjects were recruited for this study. Using the G*Power 3.1.9.7, the basis total sample size was set as 66 with 0.4 effect size, 0.05 α error probability, and 0.8 power (1−β error probability) [46]. Hence, further prospective studies are needed considering the limitations listed above.
Conclusions
In this study of 31 patients with HI-BI, we found that both the TCT and the DMN are closely associated with consciousness levels in DOC patients with HI-BI and appear to have similar impacts on consciousness levels. Our results suggest that the TCT and the DMN could be important targets for neurorehabilitation in DOC patients with HI-BI. Further studies comparing the neurological correlations of the consciousness and the specific parts of the TCT and DMN are needed.
References
1. Heinz UE, Rollnik JD, Outcome and prognosis of hypoxic brain damage patients undergoing neurological early rehabilitation: BMC Res Notes, 2015; 8; 243
2. Lu-Emerson C, Khot S, Neurological sequelae of hypoxic-ischemic brain injury: NeuroRehabilitation, 2010; 26; 35-45
3. Anderson CA, Arciniegas DB, Cognitive sequelae of hypoxic-ischemic brain injury: A review: NeuroRehabilitation, 2010; 26; 47-63
4. Lacerte M, Hays Shapshak A, Mesfin FB, Hypoxic brain injury. [Updated 2023 Jan 27]: StatPearls [Internet], 2023, Treasure Island (FL), StatPearls Publishing Available from: https://www.ncbi.nlm.nih.gov/books/NBK537310/
5. Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients: Brain, 2010; 133(Pt 1); 161-71
6. Liu X, Li J, Gao J, Association of medial prefrontal cortex connectivity with consciousness level and its outcome in patients with acquired brain injury: J Clin Neurosci, 2017; 42; 160-66
7. Jang SH, Park JS, Shin DG, Relationship between consciousness and injury of ascending reticular activating system in patients with hypoxic ischaemic brain injury: J Neurol Neurosurg Psychiatry, 2019; 90; 493-94
8. Van der Werf YD, Witter MP, Groenewegen HJ, The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness: Brain Res Brain Res Rev, 2002; 39; 107-40
9. Jang SH, Kim SH, Cho MK, Relationship between the consciousness state and the default mode network in traumatic brain injury: J Integr Neurosci, 2023; 22; 37
10. Buckner RL, Andrews-Hanna JR, Schacter DL, The brain’s default network: Anatomy, function, and relevance to disease: Ann NY Acad Sci, 2008; 1124; 1-38
11. León-Domínguez U, Vela-Bueno A, Froufé-Torres M, León-Carrión J, A chronometric functional sub-network in the thalamo-cortical system regulates the flow of neural information necessary for conscious cognitive processes: Neuropsychologia, 2013; 51; 1336-49
12. Edlow BL, Takahashi E, Wu O, Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders: J Neuropathol Exp Neurol, 2012; 71; 531-46
13. Weng L, Xie Q, Zhao L, Abnormal structural connectivity between the basal ganglia, thalamus, and frontal cortex in patients with disorders of consciousness: Cortex, 2017; 90; 71-87
14. George K, Das JM, Neuroanatomy, thalamocortical radiations. [Updated 2023 Jul 24]: StatPearls [Internet], 2023, Treasure Island (FL), StatPearls Publishing Available from: https://www.ncbi.nlm.nih.gov/books/NBK546699/
15. Jang SH, Lim HW, Yeo SS, The neural connectivity of the intralaminar thalamic nuclei in the human brain: A diffusion tensor tractography study: Neurosci Lett, 2014; 579; 140-44
16. Cavanna AE, The precuneus and consciousness: CNS Spectr, 2007; 12; 545-52
17. Parra-Morales AM, Rudas J, Vargas JA, Structural and functional connectivity of ascending reticular activating system in a patient with impaired consciousness after a cardiac arrest: A case report: Medicine (Baltimore), 2019; 98; e15620
18. Zou Q, Wu X, Hu J, Longitudinal recovery of local neuronal activity and consciousness level in acquired brain injury: Hum Brain Mapp, 2017; 38; 3579-91
19. Rosanova M, Gosseries O, Casarotto S, Recovery of cortical effective connectivity and recovery of consciousness in vegetative patients: Brain, 2012; 135(Pt 4); 1308-20
20. Yoshimura H, Togo M, Ishii J, Electroencephalographic findings in Bickerstaff’s brainstem encephalitis: A possible reflection of the dysfunction of the ascending reticular activating system: Clin Neurophysiol Pract, 2020; 6; 29-35
21. Yao S, Song J, Gao L, Thalamocortical sensorimotor circuit damage associated with disorders of consciousness for diffuse axonal injury patients: J Neurol Sci, 2015; 356; 168-74
22. Långsjö JW, Lehtimäki K, Ruohonen J, Critical neural targets for (the level of) human consciousness: Arousal arrest and unconsciousness after sumatriptan administration: Brain Inj, 2016; 30; 1731-36
23. Cunningham SI, Tomasi D, Volkow ND, Structural and functional connectivity of the precuneus and thalamus to the default mode network: Hum Brain Mapp, 2017; 38; 938-56
24. Assaf Y, Pasternak O, Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review: J Mol Neurosci, 2008; 34; 51-61
25. Mori S, Crain BJ, Chacko VP, van Zijl PC, Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging: Ann Neurol, 1999; 45; 265-69
26. Le Bihan D, Mangin JF, Poupon C, Diffusion tensor imaging: Concepts and applications: J Magn Reson Imaging, 2001; 13; 534-46
27. Jang SH, Choi EB, Relationship between the consciousness level and the structural neural connectivity of the medial prefrontal cortex in hypoxic-ischemic brain injury: A pilot study: Neuroreport, 2022; 33; 750-55
28. Jang SH, Choi EB, Differences in the thalamocortical tract of the ascending reticular activating system in disorders of consciousness after hypoxic-ischemic brain injury: A pilot study: Medicine (Baltimore), 2022; 101; e30199
29. Jang S, Choi E, Relationship between Coma Recovery Scale-Revised and the thalamocortical tract of ascending reticular activating system in hypoxic-ischemic brain injury: A pilot study: Healthcare (Basel), 2023; 11; 1148
30. Stafford CA, Owen AM, Fernández-Espejo D, The neural basis of external responsiveness in prolonged disorders of consciousness: Neuroimage Clin, 2019; 22; 101791
31. Seel RT, Sherer M, Whyte JAmerican Congress of Rehabilitation Medicine, Brain Injury-Interdisciplinary Special Interest Group, Disorders of Consciousness Task Force, Assessment scales for disorders of consciousness: evidence-based recommendations for clinical practice and research: Arch Phys Med Rehabil, 2010; 91; 1795-813
32. Gerrard P, Zafonte R, Giacino JT, Coma Recovery Scale-Revised: Evidentiary support for hierarchical grading of level of consciousness: Arch Phys Med Rehabil, 2014; 95(12); 2335-41
33. Bodien YG, Carlowicz CA, Chatelle C, Giacino JT, Sensitivity and Specificity of the Coma Recovery Scale – revised total score in detection of conscious awareness: Arch Phys Med Rehabil, 2016; 97; 490-92
34. Cohen J: Statistical power analysis for the behavioral sciences, 1988, Hillsdale, USA, Lawrence Erlbaum Associates
35. Sekhon MS, Ainslie PN, Griesdale DE, Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A “two-hit” model: Crit Care, 2017; 21; 90
36. Nolan JP, Neumar RW, Adrie C, Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation;; the American Heart Association Emergency Cardiovascular Care Committee;; the Council on Cardiovascular Surgery and Anesthesia;; the Council on Cardiopulmonary, Perioperative, and Critical Care;; the Council on Clinical Cardiology;; the Council on Stroke: Resuscitation, 2008; 79; 350-79
37. Busl KM, Greer DM, Hypoxic-ischemic brain injury: Pathophysiology, neuropathology and mechanisms: NeuroRehabilitation, 2010; 26; 5-13
38. Hossmann KA, The hypoxic brain. Insights from ischemia research: Adv Exp Med Biol, 1999; 474; 155-69
39. Howard RS, Holmes PA, Siddiqui A, Hypoxic-ischaemic brain injury: Imaging and neurophysiology abnormalities related to outcome: QJM, 2012; 105; 551-61
40. Lant ND, Gonzalez-Lara LE, Owen AM, Fernández-Espejo D, Relationship between the anterior forebrain mesocircuit and the default mode network in the structural bases of disorders of consciousness: Neuroimage Clin, 2015; 10; 27-35
41. Kawai K, Nitecka L, Ruetzler CA, Global cerebral ischemia associated with cardiac arrest in the rat: I. Dynamics of early neuronal changes: J Cereb Blood Flow Metab, 1992; 12; 238-49
42. Pulsinelli WA, Brierley JB, Plum F, Temporal profile of neuronal damage in a model of transient forebrain ischemia: Ann Neurol, 1982; 11; 491-98
43. Shoykhet M, Simons DJ, Alexander H, Thalamocortical dysfunction and thalamic injury after asphyxial cardiac arrest in developing rats: J Neurosci, 2012; 32; 4972-81
44. Cervós-Navarro J, Diemer NH, Selective vulnerability in brain hypoxia: Crit Rev Neurobiol, 1991; 6; 149-82
45. Alves PN, Foulon C, Karolis V, An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings: Commun Biol, 2019; 2; 370
46. Faul F, Erdfelder E, Lang AG, Buchner A, G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences: Behav Res Methods, 2007; 39; 175-91
Tables
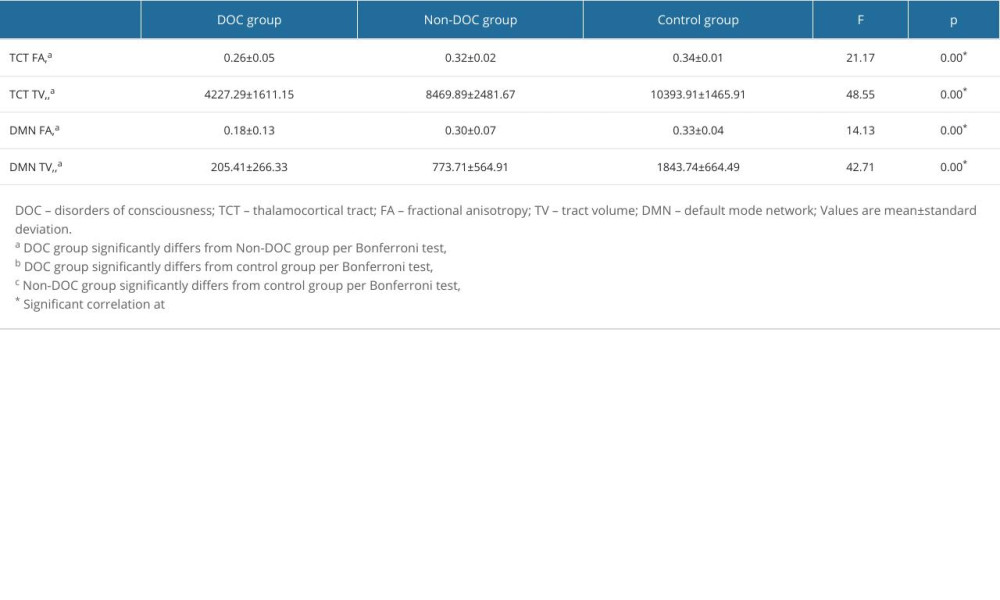 Table 1. Comparison of diffusion tensor tractography parameters of the thalamocortical tract and the default mode network among the groups.
Table 1. Comparison of diffusion tensor tractography parameters of the thalamocortical tract and the default mode network among the groups.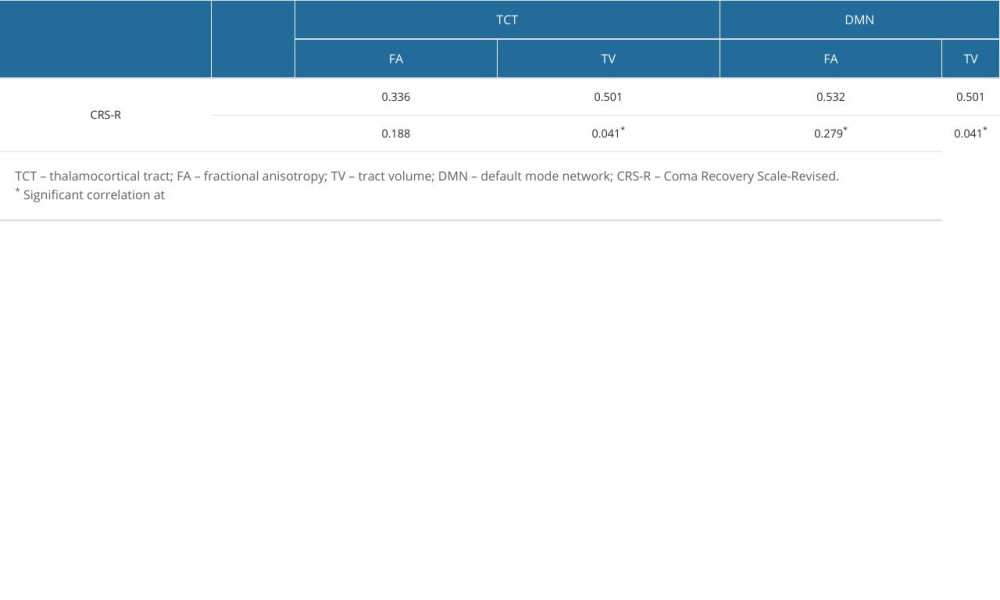 Table 2. Correlation between Coma Recovery Scale-Revised scores and diffusion tensor tractography parameters for the disorders of consciousness group.
Table 2. Correlation between Coma Recovery Scale-Revised scores and diffusion tensor tractography parameters for the disorders of consciousness group. Table 1. Comparison of diffusion tensor tractography parameters of the thalamocortical tract and the default mode network among the groups.
Table 1. Comparison of diffusion tensor tractography parameters of the thalamocortical tract and the default mode network among the groups. Table 2. Correlation between Coma Recovery Scale-Revised scores and diffusion tensor tractography parameters for the disorders of consciousness group.
Table 2. Correlation between Coma Recovery Scale-Revised scores and diffusion tensor tractography parameters for the disorders of consciousness group. In Press
16 Mar 2024 : Clinical Research
Diagnostic Efficiency of ACR-TIRADS Score for Differentiating Benign and Malignant Thyroid Nodules of Vario...Med Sci Monit In Press; DOI: 10.12659/MSM.943228
08 May 2024 : Clinical Research
Effect of Individualized PEEP Guided by Driving Pressure on Diaphragm Function in Patients Undergoing Lapar...Med Sci Monit In Press; DOI: 10.12659/MSM.944022
21 Mar 2024 : Clinical Research
Impact of Serum Vitamin D, B6, and B12 and Cognitive Functions on Quality of Life in Peri- and Postmenopaus...Med Sci Monit In Press; DOI: 10.12659/MSM.943249
17 Mar 2024 : Clinical Research
Protective Role of TRPC3 Gene Polymorphism (rs10518289) in Obstructive Sleep Apnea Hypopnea Syndrome Among ...Med Sci Monit In Press; DOI: 10.12659/MSM.942667
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952









![Diffusion tensor tractography results for representative patients in the disorders of consciousness (DOC) group [patient 1: 43-year-old female with Coma Recovery Scale-Revised (CRS-R) score 8, patient 2: 58-year-old female with CRS-R score 15], one representative patient from the non-DOC group (53-year-old male with CRS-R score 23), and one normal control subject (45-year-old male). (A) T2-weighted brain magnetic resonance images acquired at the time of diffusion tensor imaging scanning. (B) Diffusion tensor tractography results of the thalamocortical tract (TCT) and default mode network (DMN) created using the Oxford Centre for Functional Magnetic Resonance Imaging of Brain (FMRIB) Software Library (FSL, version 5.0.1, Oxford). There is a greater tract volume and integrity of the TCT and the DMN in the non-DOC patient compared to both DOC patients. Furthermore, the tract volume and integrity of the TCT and the DMN (red arrows) in patient 1 are lower than those of patient 2.](https://jours.isi-science.com/imageXml.php?i=medscimonit-30-e943802-g001.jpg&idArt=943802&w=1000)