28 March 2024: Clinical Research
Prognostic Significance of Neutrophil-to-Lymphocyte Ratio in Predicting Complete Pathological Response in Rectal Cancer Patients Receiving Neoadjuvant Chemoradiotherapy
Mustafa KaracaDOI: 10.12659/MSM.943750
Med Sci Monit 2024; 30:e943750
Abstract
BACKGROUND: Pathologic response after neoadjuvant therapy has been shown to improve outcomes in rectal cancer. Inflammatory markers, including neutrophil-to-lymphocyte ratio (NLR), have been studied to predict pathologic response and survival. This study aimed to evaluate the association between NLR and pathological response as well as outcome in patients with rectal cancer who underwent neoadjuvant chemoradiotherapy (nCRT).
MATERIAL AND METHODS: We retrospectively analyzed 187 patients with rectal cancer treated with nCRT followed by surgery between 2016 and 2020. The NLR was calculated using archival complete blood count records. Postoperative pathology reports were recorded. The NLR cut-off was determined by receiver operating characteristic curve. Kaplan-Meier survival curves and univariate and multivariate Cox regression analyses were used to analyze the relationship between NLR and clinicopathologic data to predict survival and prognosis.
RESULTS: An NLR >3.63 at diagnosis was the optimal cut-off value for predicting progression. Near-complete response rates were higher in patients with NLR <3.63 (38%) than in those with NLR >3.63 (18%) (P=0.035). The NLR <3.63 group had a significantly higher 5-year progression-free survival rate compared to the NLR >3.63 group (63.6% vs 40.1%, respectively; P=0.007). The NLR <3.63 group also had a higher 5-year overall survival (OS) rate than the NLR >3.63 group (72.3% vs 63.1%, respectively), but the difference was not statistically significant (P=0.077).
CONCLUSIONS: Our study showed a higher near-complete response rate in rectal cancer patients with NLR <3.63 receiving nCRT. This finding supports that a low preoperative NLR is a good prognostic factor in indicating pathological response.
Keywords: Lymphocytes, Neoadjuvant Therapy, Neutrophils, Rectal Neoplasms
Introduction
Colon and rectal cancers together are the third most common malignancy among men and the second among women [1]. Colorectal cancer affects approximately 150 000 estimated new patients in the United States per year and of these cases 30% are due to rectal cancer [2]. While these cancers are reported as the third leading cause of cancer-related deaths in both men and women, determining mortality due to rectal cancer alone is difficult due to the large number of deaths from rectal cancer being misclassified as colon cancer [2,3]. After pathologic confirmation and careful staging, there are strong recommendations for neoadjuvant therapy in stage II (T3 or T4 node-negative) and stage III (node-positive) so-called locally advanced disease [3]. Pathologic response after the neoadjuvant approach has been shown to reduce recurrence and improve survival in patients with locally advanced rectal cancer [4]. However, the response to neoadjuvant therapy is highly variable among patients, making it crucial to identify specific biomarkers that can predict which patients are likely to benefit and which are not.
Recent evidence increasingly recognizes cancer-associated systemic inflammation as a significant determinant of survival [5]. Various studies have demonstrated that the neutrophil-to-lymphocyte ratio (NLR), a hematologic inflammatory marker, could be a predictive marker for oncological outcomes and response to neoadjuvant chemoradiotherapy (nCRT) in patients with rectal cancer [6–9]. In a recent meta-analysis of 22 studies comprising 6316 patients, elevated pre-nCRT NLR was found to be strongly associated with worse treatment outcomes in patients with rectal cancer [10]. Although many studies have explored the correlation between NLR and outcome, few have examined the relationship between NLR and pathologic responses. Therefore, this retrospective study from a single center in Turkey included 187 patients with rectal cancer who underwent nCRT, and aimed to evaluate the association between NLR and pathologic response as well as outcome.
Material and Methods
ETHICS STATEMENT:
The study was conducted according to the standards of the Helsinki Declaration and approval was obtained from the Health Sciences University Antalya Training and Research Hospital’s clinical research ethics committee on 30.09.2021, with decision number 15/19.
PATIENTS:
Our study included 187 patients with rectal cancer treated at the medical oncology department of the Health Sciences University Antalya Training and Research Hospital between 2016 and 2020. All patients had histologically confirmed stage III rectal adenocarcinoma and underwent surgery after approximately 4 to 6 weeks of nCRT followed by a 4-to-8-week interval.
DATA COLLECTION:
Demographic, clinicopathologic, and laboratory data of the patients were retrospectively recorded using archival files and hospital database. Absolute neutrophil (normal: 2–7.15×103/mm3) and lymphocyte (normal: 1.16–3.18×103/mm3) values were extracted from hospital computer system complete blood count results within 2 weeks before nCRT initiation and after surgery. NLR was calculated as the absolute number of neutrophils divided by the absolute number of lymphocytes. Postoperative pathology reports were also evaluated from archive files to record pathologic response to nCRT.
PATHOLOGIC RESPONSE CRITERIA AND STUDY OUTCOMES:
The degree of primary tumor regression was assessed in 4 categories using the modified RYAN scheme, as recommended by the College of American Pathologists [11]: complete response (no viable cancer cells), near-complete response (single cells or rare small groups of cancer cells), partial response (residual cancer with evident tumor regression, but more than single cells or rare small groups of cancer cells), and poor or no response (extensive residual cancer with no evident tumor regression). Progression-free survival (PFS) was calculated from surgery to local or distant failure or death. Overall survival (OS) was defined as the time from initial diagnosis to death from any cause or date of last known contact.
STATISTICAL ANALYSIS:
For categorical variables, descriptive statistics are presented as frequency (n) and percentage (%), and for continuous variables as mean, standard deviation (SD), median, and interquartile range (IQR: 25th–75th percentile). Relationships between categorical variables were examined using the Pearson chi-square test and Fisher’s exact test. The assumption of normal distribution was checked with the Shapiro-Wilk test. Non-parametric comparisons of continuous variables between independent groups were analyzed using the Mann-Whitney U test and Kruskal-Wallis test, while parametric comparisons were conducted with the independent
To differentiate among patients based on NLR values and to determine a cut-off point in predicting progression, receiver operating characteristic (ROC) analysis was conducted. The results of the analysis were presented with the area under the curve (AUC), cut-off points, sensitivity and specificity values, and 95% confidence intervals (CI). The optimal cut-off point for NLR was calculated using the Youden index.
Survival curves were generated using the Kaplan-Meier method, and OS and PFS rates according to NLR groups were compared using the log-rank test. Factors affecting OS and PFS were evaluated using univariate and multivariate Cox regression analyses. Variables with a
The study sample size was calculated using the G*Power 3.1.9 program based on the effect size determined from the study of Kim et al [9]. All statistical analyses were performed using IBM SPSS 23.0 software package (IBM Corp., Armonk, NY) and P values less than 0.05 were considered statistically significant.
Results
PATIENT CHARACTERISTICS AND TREATMENT:
The demographic, clinicopathological data, and treatment responses of the patients are summarized in Table 1. The study included 187 patients with an average age of 64.59±11.19, of which 65.8% were male. During chemoradiotherapy, 51.3% of the patients received capecitabine. The most common surgical procedure performed was low anterior resection (LAR) (82.4%), and pathological complete response was achieved in 34 patients (18.2%) after nCRT.
PRE-NCRT AND POSTOPERATIVE LABORATORY PARAMETERS:
Descriptive statistics for the biochemical parameters of the patients at diagnosis and in the postoperative period are provided in Table 2.
ROC CURVE FOR RECURRENCE PREDICTION AND PATIENT CHARACTERISTICS ACCORDING TO NLR CUT-OFF:
In the ROC analysis conducted to determine the discriminative performance of the NLR parameter at diagnosis in predicting progression, the cut-off value was determined to be 3.63 (AUC=0.609 [95% CI: 0.535–0.679]; P=0.011) (Figure 1). Having an NLR threshold level >3.63 at diagnosis could predict PFS with 39.44% sensitivity and 81.9% specificity.
The general characteristics of the patients divided into 2 groups according to the NLR cut-off value are compared in Table 3. When the treatment response rates of the 2 groups were analyzed, the near-complete response rate was higher in patients with NLR <3.63 (38% vs 18%; P=0.035). The poor response rate was significantly higher in the group with NLR >3.63 (64% vs 43.8%; P=0.014).
PATIENT CHARACTERISTICS ACCORDING TO PATHOLOGICAL RESPONSE:
Table 4 compares the general characteristics of patients according to pathologic response after treatment. There was no significant difference between the hemoglobin (P=0.247), neutrophil (P=0.198), lymphocyte (P=0.580), platelet (P=0.903), and lactate dehydrogenase (LDH) (P=0.756) values at the time of diagnosis. Although NLR values were higher in patients with partial response and patients with no response, this difference was not statistically significant (P=0.127). The rate of NLR >3.63 in patients with no response to treatment (41.7%) was significantly higher than in patients with near-complete response (14.8%) (P=0.040).
PROGNOSTIC FACTORS:
A multivariate logistic regression analysis was performed to determine the risk factors independently associated with poor pathologic response in the patients included in the study, and the results are presented in Table 5. Poor pathological response was found to be associated with younger ages (P=0.006) and with NLR >3.63 at diagnosis (P=0.012).
The 5-year PFS rate was 63.6% in the NLR <3.63 group and 40.1% in the NLR >3.63 group. PFS rates were significantly lower in the group with NLR >3.63 (P=0.007) (Figure 2). The 5-year OS rate was 72.3% in the NLR <3.63 group and 63.1% in the NLR >3.63 group. Although the OS rates were lower in the NLR >3.63 group, this difference was not statistically significant (P=0.077) (Figure 3).
The risk factors affecting PFS in patients were evaluated by univariate and multivariate Cox regression analysis and are shown in Table 6. According to univariate analysis, PFS was associated with sex, the type of chemotherapy drug used, and NLR >3.63 at the time of diagnosis. Multivariate analysis showed that the risk of poor PFS was higher in patients who received 5-Fluorouracil (5-FU) as a chemotherapy agent (P=0.011).
The results of univariate and multivariate Cox regression analysis to determine the risk factors affecting OS in patients are presented in Table 7. In univariate analyses, age, chemotherapy agent used, and type of surgery were associated with OS, while decreasing age (P=0.017), 5-FU use (P=0.020), and MILES operation (P=0.025) negatively affected OS.
Discussion
Neoadjuvant therapy for rectal cancer is used to down-stage tumors, reduce local recurrences, and improve survival. Pathologic response after nCRT has been shown to reduce recurrence and improve survival in patients [4,12]. Inflammatory response plays a significant role in tumor development, progression, immune surveillance, and response to treatment. NLR is a cost-effective, easily accessible inflammatory parameter derived from peripheral blood count, which can predict prognosis, unlike traditional risk markers. The prognostic role of NLR has been demonstrated in many solid tumors, as shown in a recent meta-analysis including 100 studies [13]. Pre-treatment high NLR has been correlated with poor response to treatment, adverse pathological outcomes, and poor survival [14]. In our study, low preoperative NLR (<3.63) was found to be a good prognostic factor in indicating pathological response, and high NLR (>3.63) was associated with worse PFS.
In our study of 187 patients receiving nCRT, the mean age was 64.59±11.19 years, and 65.8% were male. In a study conducted by Nagasaki et al involving 201 patients, 52.2% were over 60 years old and 68.9% were male [15]. The sex and average age of patients in our study are consistent with the literature.
Regarding the chemotherapy agent used during nCRT, Kim et al [9] reported that 31.3% of patients received capecitabine, while Nagasaki et al [15] included only patients receiving 5-FU. Our study is more suitable for comparing responses to both chemotherapy agents.
Similar to the rate of complete responders in our study, in the study by Nagasaki et al [15], 26 patients (12.9%) had complete response and 175 patients (87.1%) were non-responsive.
The NLR cut-off value in our study is consistent with previous studies in the literature [9,15,16]. The variation in NLR values within a certain range can be attributed to the different patient numbers included in the studies and the inclusion of patients with different stages of rectal cancer. In our study, consistent with the literature [15,17], patients with high NLR were significantly older than those with low NLR. The literature shows that high NLR is generally associated with older average age. In the study by Nagasaki et al [15], similar to our study, the high NLR group had a higher proportion of male patients, while Dimitriou et al [17] included more patients and found an equal sex distribution in the high NLR group, contrary to our findings.
In our study addressing the relationship between NLR and pathological response, patients were categorized into 4 groups: complete response, near-complete response, partial response, and no response. Examining the treatment response rates according to NLR groups, a higher rate of near-complete response was observed in patients with NLR <3.63 (38% vs 18%;
No significant difference was observed between the age averages (
In our study and in the study conducted by Kim et al [9], no significant differences were found in the hemoglobin (
In our study, the proportion of patients with NLR >3.63 was significantly higher in the group without treatment response (41.7%) compared to the group with near-complete response (14.8%) (
In our study, the 5-year PFS was significantly lower in the group with NLR >3.63 (
In univariate analyses, sex (
In our study, although the OS rates were lower in the group with NLR >3.63, this difference was not statistically significant (
Univariate analyses conducted to identify risk factors affecting patients’ OS revealed that age (
In the multivariate analysis for OS, younger age (
A limitation of the present study is that it was a retrospective, single-center study, but its strengths include having a sample size similar to those of other studies and the comparison of factors that were not compared in similar studies in the literature (comparison of patients according to hemoglobin, platelet, and LDH values according to NLR groups). To the best of our knowledge, there have been no previous studies that evaluated the distribution of comorbid diseases and the relationship between the type of operation and pathological response.
Conclusions
In our study, a higher rate of near-complete response was observed in rectal cancer patients with NLR <3.63 receiving nCRT. This supports, in line with the literature, that a low preoperative NLR ratio is a good prognostic factor in indicating pathological response.
Figures
![ROC curve for NLR in predicting PFS. The cut-off value was determined to be 3.63 (AUC=0.609 [95% CI: 0.535–0.679]; P=0.011). NLR threshold level >3.63 at diagnosis could predict PFS with 39.44% sensitivity and 81.9% specificity. AUC – the area under the curve; NLR – neutrophil-to-lymphocyte ratio; ROC – receiver operating characteristic; PFS – progression-free survival. We used SPSS 23.0 software package (IBM Corp., Armonk, NY) to draw this figure.](https://jours.isi-science.com/imageXml.php?i=medscimonit-30-e943750-g001.jpg&idArt=943750&w=1000) Figure 1. ROC curve for NLR in predicting PFS. The cut-off value was determined to be 3.63 (AUC=0.609 [95% CI: 0.535–0.679]; P=0.011). NLR threshold level >3.63 at diagnosis could predict PFS with 39.44% sensitivity and 81.9% specificity. AUC – the area under the curve; NLR – neutrophil-to-lymphocyte ratio; ROC – receiver operating characteristic; PFS – progression-free survival. We used SPSS 23.0 software package (IBM Corp., Armonk, NY) to draw this figure.
Figure 1. ROC curve for NLR in predicting PFS. The cut-off value was determined to be 3.63 (AUC=0.609 [95% CI: 0.535–0.679]; P=0.011). NLR threshold level >3.63 at diagnosis could predict PFS with 39.44% sensitivity and 81.9% specificity. AUC – the area under the curve; NLR – neutrophil-to-lymphocyte ratio; ROC – receiver operating characteristic; PFS – progression-free survival. We used SPSS 23.0 software package (IBM Corp., Armonk, NY) to draw this figure. 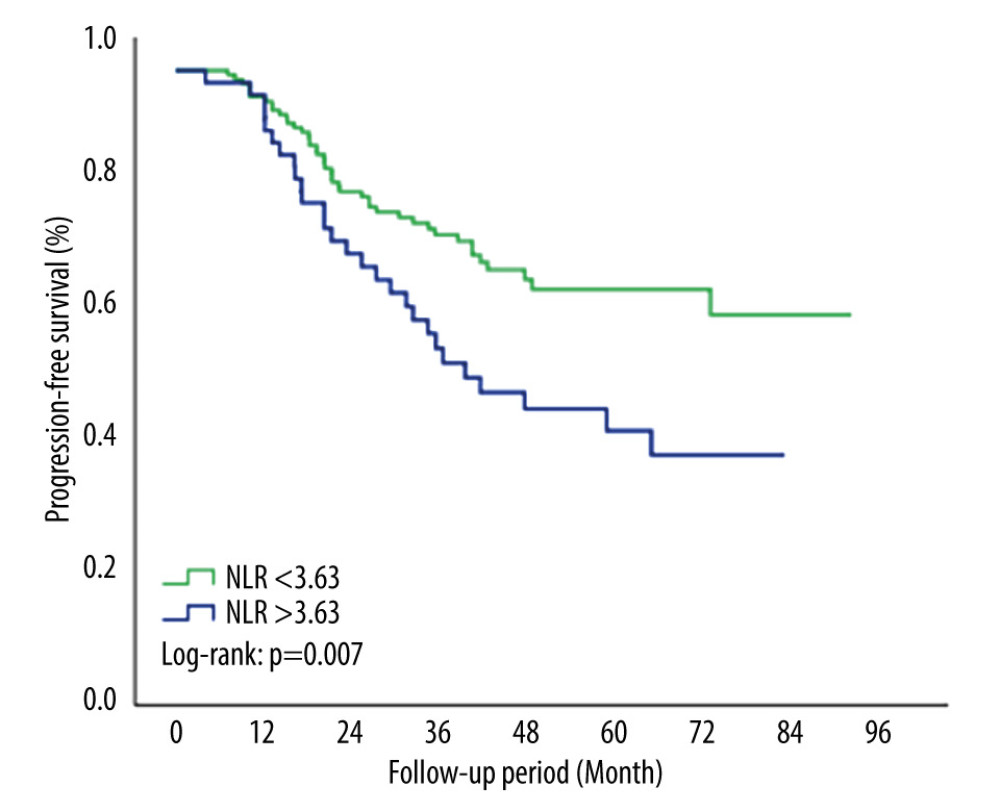 Figure 2. Kaplan-Meier survival curve for PFS by NLR groups. Patients with NLR >3.63 had significantly worse PFS rates compared with NLR <3.63 (P=0.007). NLR – neutrophil-to-lymphocyte ratio; PFS – progression-free survival. We used SPSS 23.0 software package (IBM Corp., Armonk, NY) to draw this figure.
Figure 2. Kaplan-Meier survival curve for PFS by NLR groups. Patients with NLR >3.63 had significantly worse PFS rates compared with NLR <3.63 (P=0.007). NLR – neutrophil-to-lymphocyte ratio; PFS – progression-free survival. We used SPSS 23.0 software package (IBM Corp., Armonk, NY) to draw this figure. 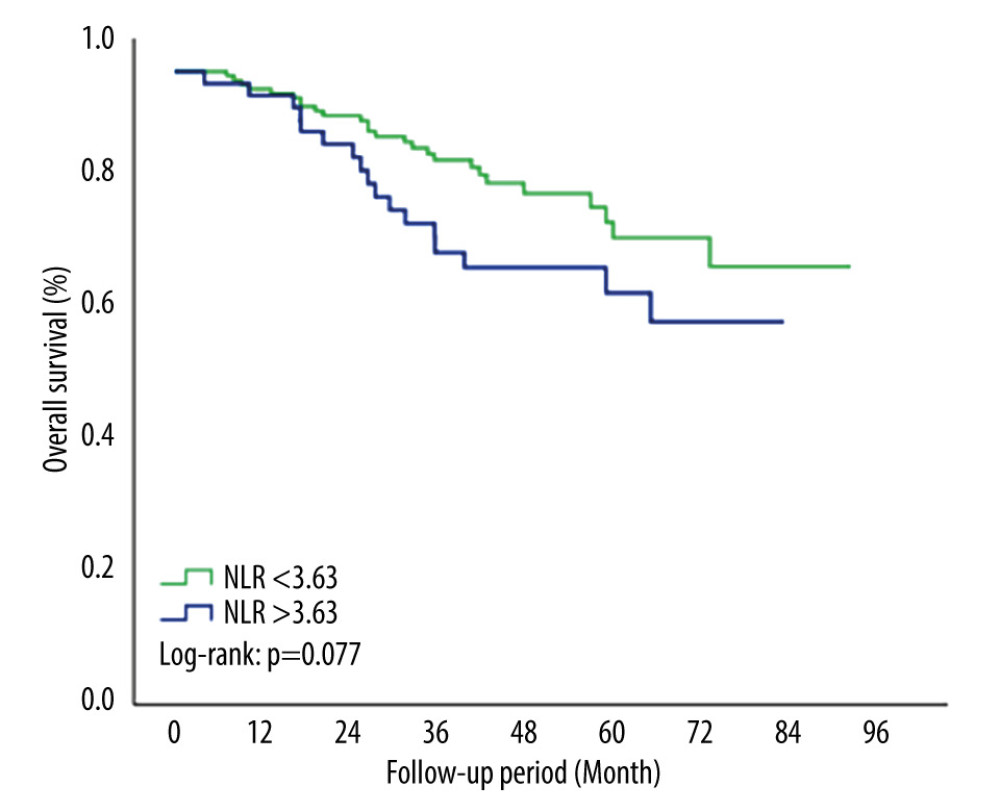 Figure 3. Kaplan-Meier survival curve for OS by NLR groups. The 5-year OS rates were lower in the NLR >3.63 group but this difference was not statistically significant (P=0.077). NLR – neutrophil-to-lymphocyte ratio; OS – overall survival. We used SPSS 23.0 software package (IBM Corp., Armonk, NY) to draw this figure.
Figure 3. Kaplan-Meier survival curve for OS by NLR groups. The 5-year OS rates were lower in the NLR >3.63 group but this difference was not statistically significant (P=0.077). NLR – neutrophil-to-lymphocyte ratio; OS – overall survival. We used SPSS 23.0 software package (IBM Corp., Armonk, NY) to draw this figure. Tables
Table 1. Demographic and clinical characteristics of the patients.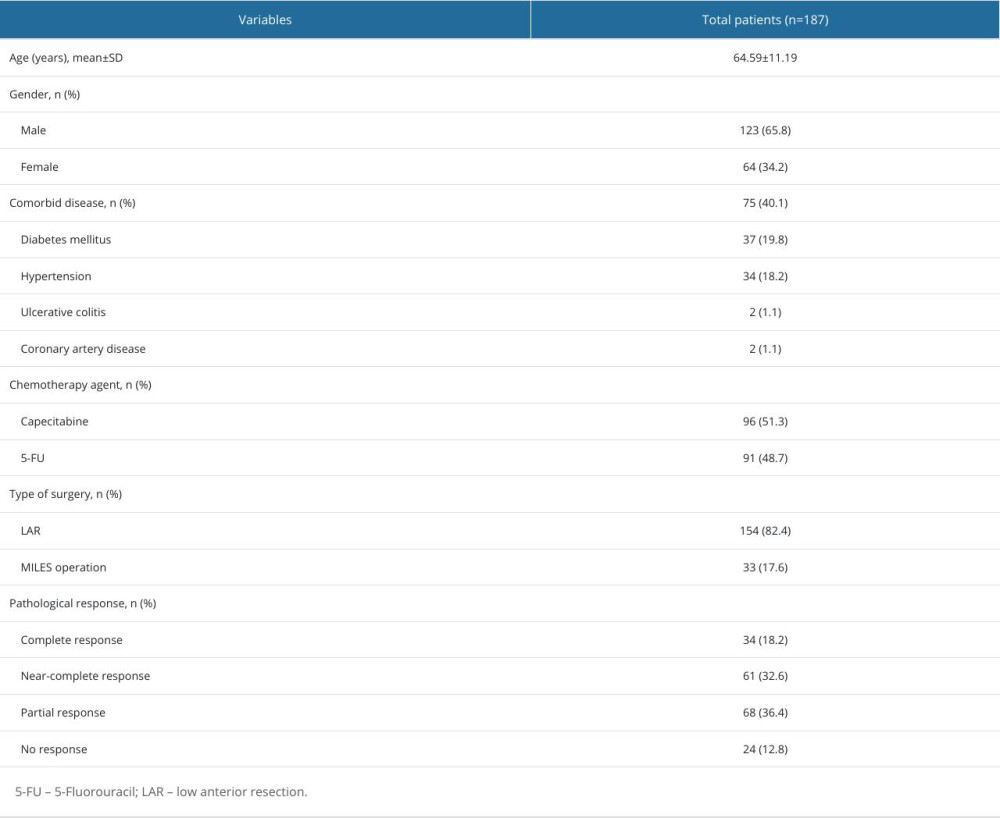 Table 2. Biochemical parameters of patients.
Table 2. Biochemical parameters of patients.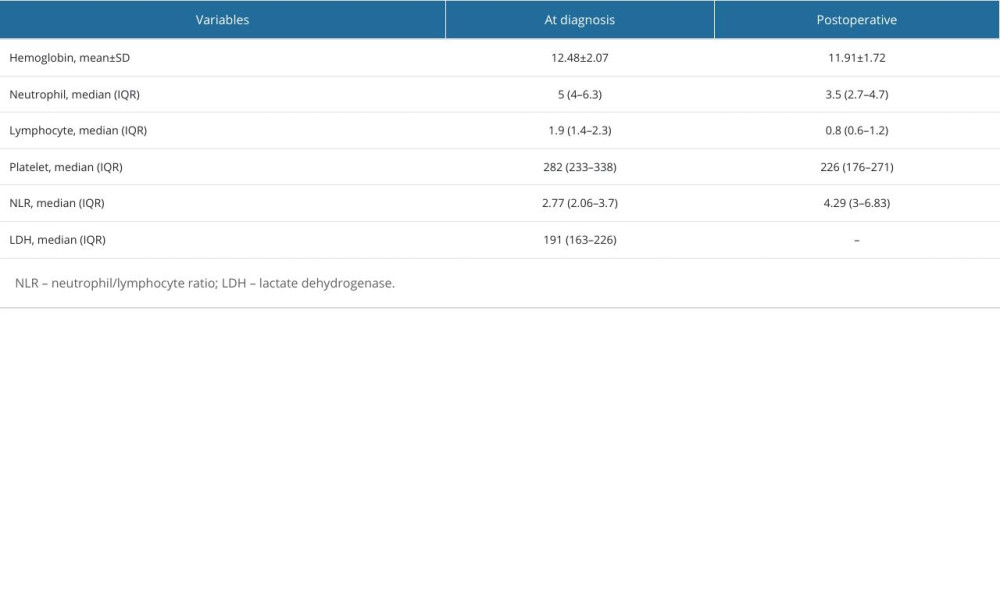 Table 3. Patient characteristics according to NLR groups.
Table 3. Patient characteristics according to NLR groups.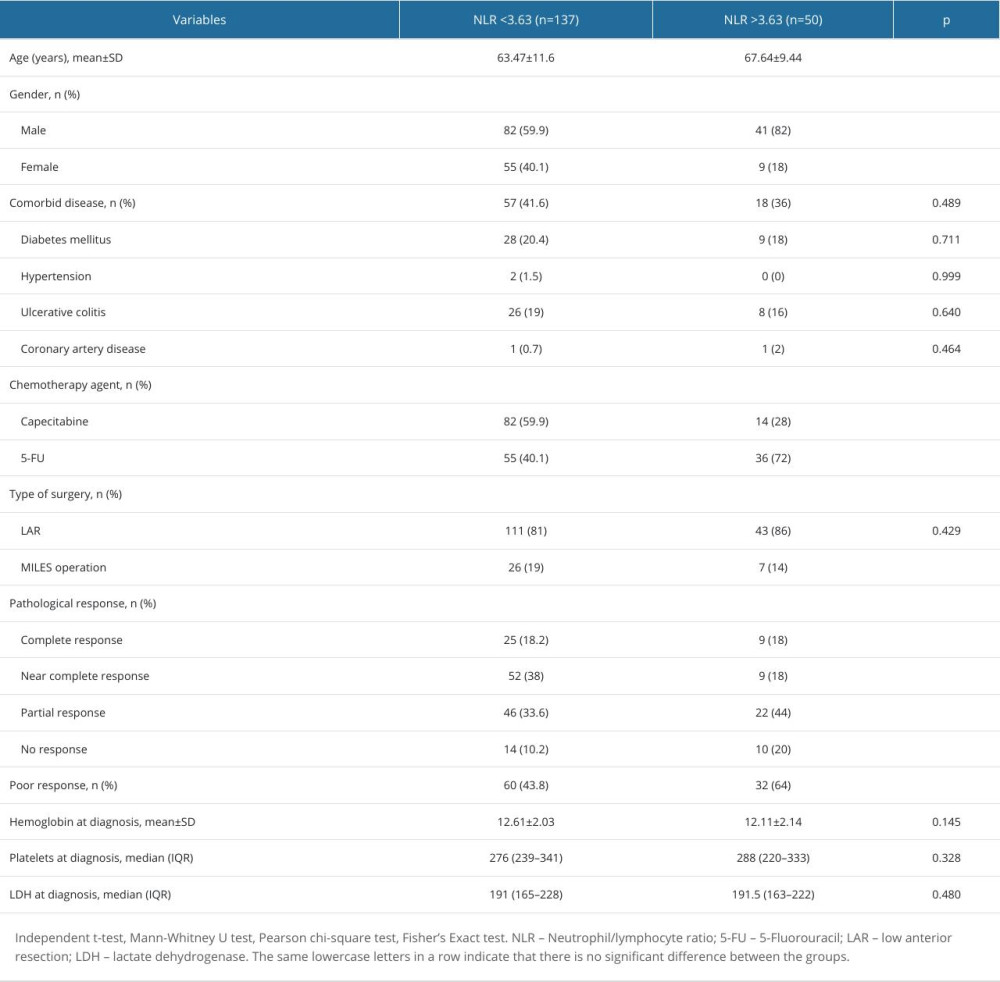 Table 4. Patient characteristics according to pathological response.
Table 4. Patient characteristics according to pathological response.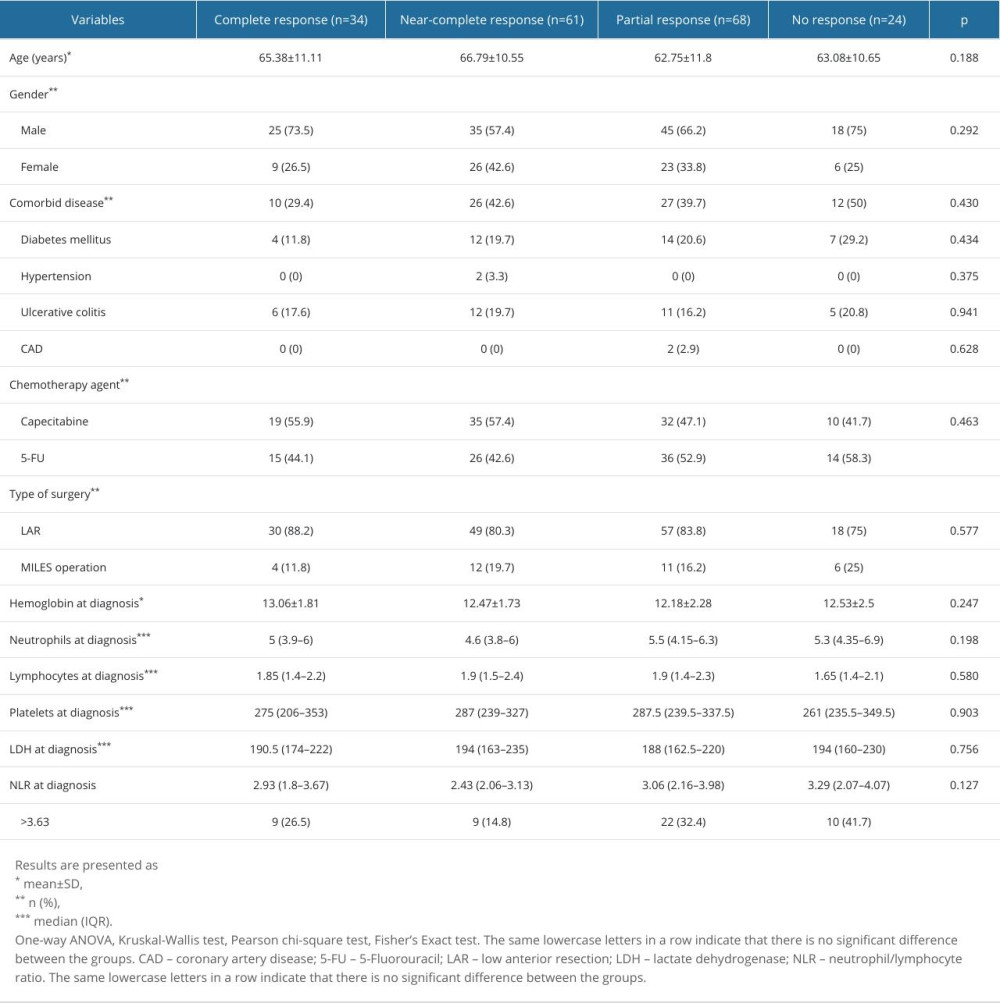 Table 5. Factors associated with poor pathologic response.
Table 5. Factors associated with poor pathologic response.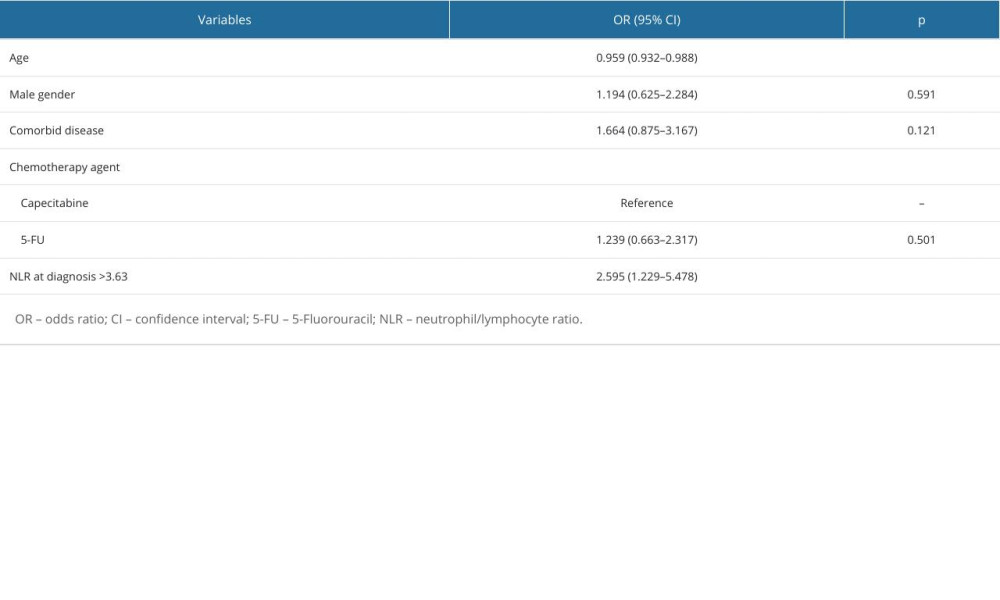 Table 6. Factors associated with progression-free survival of patients.
Table 6. Factors associated with progression-free survival of patients.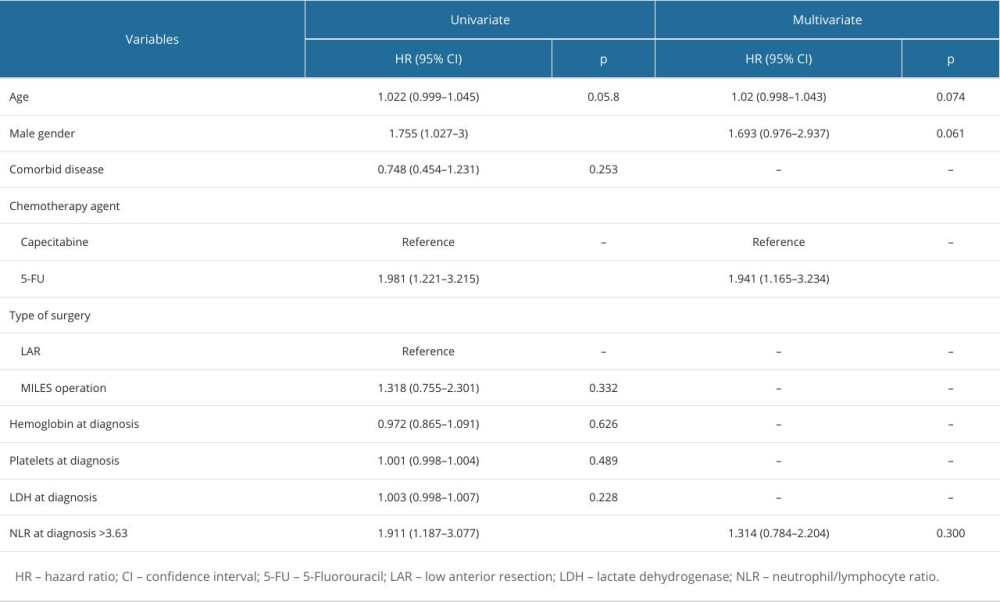 Table 7. Factors associated with overall survival of patients.
Table 7. Factors associated with overall survival of patients.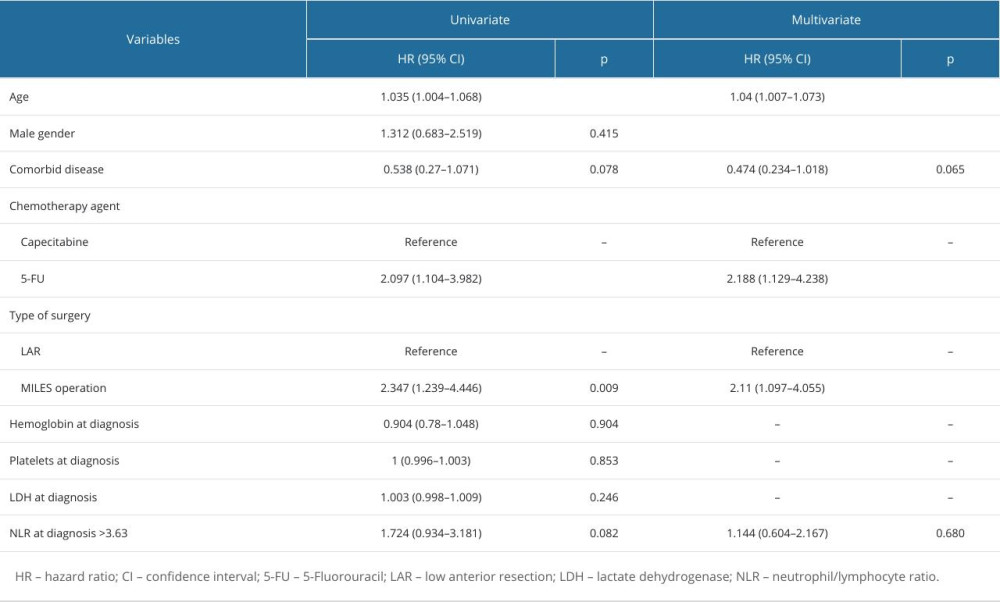
References
1. Sung H, Ferlay J, Siegel RL, Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2021; 71(3); 209-49
2. Siegel RL, Miller KD, Wagle NS, Jemal A, Cancer statistics, 2023: Cancer J Clin, 2023; 73(1); 17-48
3. Lotfollahzadeh S, Kashyap S, Tsoris A, Rectal cancer. [Updated 2023 Jul 4]: StatPearls [Internet], 2024, Treasure Island (FL), StatPearls Publishing Available from: https://www.ncbi.nlm.nih.gov/books/NBK493202/
4. Martin ST, Heneghan HM, Winter DC, Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer: Br J Surg, 2012; 99(7); 918-28
5. Roxburgh CS, Salmond JM, Horgan PG, The relationship between the local and systemic inflammatory responses and survival in patients undergoing curative surgery for colon and rectal cancers: J Gastrointest Surg, 2009; 13(11); 2011-18 discussion 2018–19
6. Shen J, Zhu Y, Wu W, Prognostic role of neutrophil-to-lymphocyte ratio in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy: Med Sci Monit, 2017; 23; 315-24
7. Zhang X, Li J, Peng Q, Association of markers of systemic and local inflammation with prognosis of patients with rectal cancer who received neoadjuvant radiotherapy: Cancer Manag Res, 2019; 11; 191-99
8. Hodek M, Sirák I, Ferko A, Neoadjuvant chemoradiotherapy of rectal carcinoma: Baseline hematologic parameters influencing outcomes: Strahlenther Onkol, 2016; 192(9); 632-40
9. Kim TG, Park W, Kim H, Baseline neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in rectal cancer patients following neoadjuvant chemoradiotherapy: Tumori, 2019; 105(5); 434-40
10. Lee SJ, Kim K, Park HJ, Meta-analysis on the neutrophil-lymphocyte ratio in rectal cancer treated with preoperative chemoradiotherapy: prognostic value of pre- and post-chemoradiotherapy neutrophil-lymphocyte ratio: Front Oncol, 2022; 12; 778607
11. Ryan R, Gibbons D, Hyland JM, Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer: Histopathology, 2005; 47(2); 141-46
12. de Campos-Lobato LF, Stocchi L, da Luz Moreira A, Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence: Ann Surg Oncol, 2011; 18(6); 1590-98
13. Templeton AJ, McNamara MG, Šeruga B, Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis: J Natl Cancer Inst, 2014; 106(6); dju124
14. Li X, Dai D, Chen B, The value of neutrophil-to-lymphocyte ratio for response and prognostic effect of neoadjuvant chemotherapy in solid tumors: A systematic review and meta-analysis: J Cancer, 2018; 9(5); 861-71
15. Nagasaki T, Akiyoshi T, Fujimoto Y, Prognostic impact of neutrophil-to-lymphocyte ratio in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy: Dig Surg, 2015; 32(6); 496-503
16. Yoshida D, Minami K, Sugiyama M, Prognostic impact of the neutrophil-to-lymphocyte ratio in stage I–II rectal cancer patients: J Surg Res, 2020; 245; 281-87
17. Dimitriou N, Felekouras E, Karavokyros I, Neutrophils to lymphocytes ratio as a useful prognosticator for stage II colorectal cancer patients: BMC Cancer, 2018; 18(1); 1202
Figures
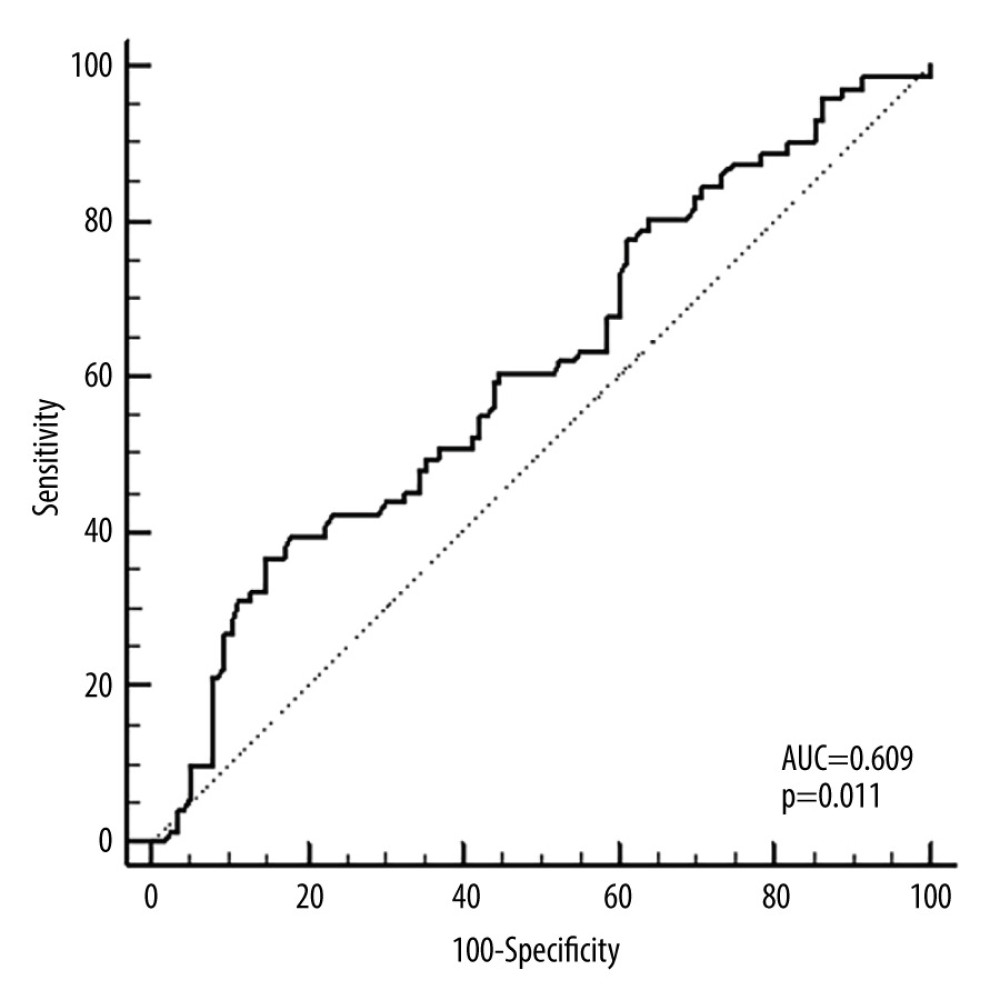 Figure 1. ROC curve for NLR in predicting PFS. The cut-off value was determined to be 3.63 (AUC=0.609 [95% CI: 0.535–0.679]; P=0.011). NLR threshold level >3.63 at diagnosis could predict PFS with 39.44% sensitivity and 81.9% specificity. AUC – the area under the curve; NLR – neutrophil-to-lymphocyte ratio; ROC – receiver operating characteristic; PFS – progression-free survival. We used SPSS 23.0 software package (IBM Corp., Armonk, NY) to draw this figure.
Figure 1. ROC curve for NLR in predicting PFS. The cut-off value was determined to be 3.63 (AUC=0.609 [95% CI: 0.535–0.679]; P=0.011). NLR threshold level >3.63 at diagnosis could predict PFS with 39.44% sensitivity and 81.9% specificity. AUC – the area under the curve; NLR – neutrophil-to-lymphocyte ratio; ROC – receiver operating characteristic; PFS – progression-free survival. We used SPSS 23.0 software package (IBM Corp., Armonk, NY) to draw this figure. Figure 2. Kaplan-Meier survival curve for PFS by NLR groups. Patients with NLR >3.63 had significantly worse PFS rates compared with NLR <3.63 (P=0.007). NLR – neutrophil-to-lymphocyte ratio; PFS – progression-free survival. We used SPSS 23.0 software package (IBM Corp., Armonk, NY) to draw this figure.
Figure 2. Kaplan-Meier survival curve for PFS by NLR groups. Patients with NLR >3.63 had significantly worse PFS rates compared with NLR <3.63 (P=0.007). NLR – neutrophil-to-lymphocyte ratio; PFS – progression-free survival. We used SPSS 23.0 software package (IBM Corp., Armonk, NY) to draw this figure. Figure 3. Kaplan-Meier survival curve for OS by NLR groups. The 5-year OS rates were lower in the NLR >3.63 group but this difference was not statistically significant (P=0.077). NLR – neutrophil-to-lymphocyte ratio; OS – overall survival. We used SPSS 23.0 software package (IBM Corp., Armonk, NY) to draw this figure.
Figure 3. Kaplan-Meier survival curve for OS by NLR groups. The 5-year OS rates were lower in the NLR >3.63 group but this difference was not statistically significant (P=0.077). NLR – neutrophil-to-lymphocyte ratio; OS – overall survival. We used SPSS 23.0 software package (IBM Corp., Armonk, NY) to draw this figure. Tables
 Table 1. Demographic and clinical characteristics of the patients.
Table 1. Demographic and clinical characteristics of the patients. Table 2. Biochemical parameters of patients.
Table 2. Biochemical parameters of patients. Table 3. Patient characteristics according to NLR groups.
Table 3. Patient characteristics according to NLR groups. Table 4. Patient characteristics according to pathological response.
Table 4. Patient characteristics according to pathological response. Table 5. Factors associated with poor pathologic response.
Table 5. Factors associated with poor pathologic response. Table 6. Factors associated with progression-free survival of patients.
Table 6. Factors associated with progression-free survival of patients. Table 7. Factors associated with overall survival of patients.
Table 7. Factors associated with overall survival of patients. Table 1. Demographic and clinical characteristics of the patients.
Table 1. Demographic and clinical characteristics of the patients. Table 2. Biochemical parameters of patients.
Table 2. Biochemical parameters of patients. Table 3. Patient characteristics according to NLR groups.
Table 3. Patient characteristics according to NLR groups. Table 4. Patient characteristics according to pathological response.
Table 4. Patient characteristics according to pathological response. Table 5. Factors associated with poor pathologic response.
Table 5. Factors associated with poor pathologic response. Table 6. Factors associated with progression-free survival of patients.
Table 6. Factors associated with progression-free survival of patients. Table 7. Factors associated with overall survival of patients.
Table 7. Factors associated with overall survival of patients. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








